AbstractObjectivesThis study aimed to investigate the prevalence, incidence, and real-world diagnostic and treatment patterns, healthcare resource utilization (HCRU), and associated costs of narcolepsy in Germany.
MethodsThis study was based on German claims data (2013–2018). Any patient with at least two outpatient specialist diagnoses and/or one inpatient diagnosis of narcolepsy was eligible for inclusion. Three cohorts were specified: 1) narcolepsy-prevalent patients alive on July 1, 2017; 2) narcolepsy-incident patients; and 3) newly treated patients. Descriptive analyses of the outcome measures were conducted.
ResultsWe identified 133 prevalent narcolepsy patients (mean age: 46.2 years, 36.3% female), 71 incident patients, and 41 treatment starters. The prevalence of narcolepsy was 3.1–9.1 per 100,000 persons within the German population; the cumulative incidence between July 1, 2017, and June 30, 2018, was 0.83/100,000 persons. Among the incident patients, 62.0% underwent at least one predefined diagnostic procedure. Modafinil was the most prescribed medication for the treatment starters (46.3%) and prevalent patients (24.1%), but 59.4% of the prevalent patients did not receive any narcolepsy-specific pharmacological treatment. Prevalent patients with narcolepsy consulted physicians significantly more often than a healthy matched control group and experienced more all-cause hospitalizations. The mean total direct healthcare costs were higher for narcolepsy patients by €2,429 per patient-year.
INTRODUCTIONNarcolepsy is a chronic sleep disorder characterized by excessive daytime sleepiness (EDS) and the dysregulation of rapid eye movement sleep [1,2]. Patients commonly experience hypnagogic and hypnopompic hallucinations, sleep paralysis, and disturbed night sleep. It is estimated that in 40%–50% of patients, narcolepsy initially manifests in conjunction with cataplexy [2,3], which refers to sudden muscle paralysis triggered by intensely positive or negative emotions [4]. This condition is classified as narcolepsy type 1 (NT1) according to the new International Classification of Sleep Disorders [5], while narcolepsy type 2 (NT2) refers to narcolepsy without cataplexy [3]. The onset of both types can occur at any life stage, with the initial diagnosis showing two main distribution peaks at 10–20 and 30–45 years of age [6].
Narcolepsy results in a great burden for affected patients; it is associated with negative effects on mental health and quality of life [7], altered executive control, attention deficits [8], and vehicle accidents [9]. Narcolepsy-related EDS is associated with symptoms like tiredness, resistible and irresistible sleepiness, and sudden sleep attacks, which lead to occupational problems for several patients [10]. Narcolepsy is often accompanied by psychiatric comorbidities, including mood disorders, depression, and anxiety [11,12], and other comorbidities, such as obesity [11,13], cardiovascular diseases [13], and other sleep disorders [14].
Treatment for narcolepsy consists of pharmacological therapies as well as behavioral and lifestyle changes [15]. Adjustments in behavior and lifestyle include regular sleep schedules, scheduled daytime naps, dietary changes, and preventing sleep deprivation [1,16]. The majority of patients additionally require pharmacological treatment to manage the main symptoms of narcolepsy. The psychostimulants modafinil, methylphenidate, and, recently, solriamfetol are approved medications for EDS in Germany, while sodium oxybate and the antidepressant clomipramine are approved for the treatment of narcolepsy with the NT1 [17,18]. Pitolisant is approved for both NT1 and NT2 conditions. Additionally, it is known that several antidepressants may be prescribed off-label to treat cataplexy; examples include venlafaxine, fluoxetine, reboxetine, and citalopram [17]. Ephedrine, dextroamphetamine, and monoamine oxidase (MAO) inhibitors are recommended as second-line therapy for EDS. Benzodiazepines may be used to treat other symptoms of narcolepsy, including hallucinations and fragmented sleep [16]. Generally, guidelines recommend that pharmacological treatment decisions should be based on clinical considerations, including the type of narcolepsy, comorbid conditions, and specific patient needs [18,19]. As the management of long-term narcolepsy requires regular evaluation and may include frequent drug switching, dose adjustments, and combination therapies, it poses a challenge to treating physicians [1,19].
There is a lack of epidemiological and real-world treatment data on patients with narcolepsy in Germany, especially regarding the current prevalence and incidence, measures related to the diagnosis of patients, their treatment as well as healthcare resource utilization (HCRU), and costs associated with this disease. We conducted this retrospective claims data study to reduce the current knowledge gap.
METHODSData source and sample selectionThis retrospective claims data analysis was based on an anonymized dataset of 3.4 million insured individuals provided by the regional German statutory health insurance fund AOK PLUS, representing 4.7% of the statutorily insured German population. The dataset contained data on the demographics (age, sex, level of care, receipt of a pension, date of death), outpatient treatment (visits to general practitioners [GPs] and specialists identified via physician code [Arztgruppenschlüssel, AGS code], diagnostic procedures, diagnosis codes, cost based on documented activity points, non-medicinal therapy), inpatient treatment (hospitalizations, diagnostic procedures, main diagnoses, length of stay, rehabilitation days, costs), outpatient medication (anatomic therapeutic classification [ATC] codes for medication identification, date of prescription, daily defined dose [DDD] costs), and disease burden (e.g., days absent from work).
The dataset covered five years from July 1, 2013 to June 30, 2018. The requirements for general inclusion in the study were two outpatient diagnoses of narcolepsy (The International Statistical Classification of Diseases And Related Health Problems, 10th revision, German Modification-ICD-10-GM code: G47.4) by a narcolepsy specialist (AGS codes 51, 53, 58, 60, 61, 44, 47, 30, 45, or 40 including neurology, physiotherapy, psychiatry, neuropediatrics, pediatric psychiatry and psychotherapy, pulmonology, and pediatrics) in two distinct quarters and/or at least one inpatient diagnosis as well as continuous insurance with the health fund throughout the entire study period (death as the only acceptable exception).
Three distinctive patient samples that were not mutually exclusive were discovered (Table 1). Sample 1 (narcolepsy-prevalent) consisted of all narcolepsy-prevalent patients who were alive on July 1, 2017. These patients were observed regarding their treatment and HCRU/cost from July 1, 2017 to June 30, 2018, or until death, whichever came first. During the sensitivity analysis, we re-defined sample 1 to include patients who received two outpatient narcolepsy diagnoses (ICD-10-GM: G47.4) by a GP (AGS codes 1, 2, 3, 34, 38, or 39) in two distinct quarters during the study period, but not by any specialist.
Sample 2 (narcolepsy incident) received their first narcolepsy diagnoses between July 1, 2014 and June 30, 2017, without any previous narcolepsy diagnosis since July 1, 2013. Sample 3 (treatment-naïve) started their first narcolepsy-specific pharmacological therapy between July 1, 2014 and June 30, 2017, and had not received any narcolepsy-specific medication for at least 12 months earlier. Based on a guideline and clinical expert review, the following medications were considered as narcolepsy-specific: nervous system drugs (sodium oxybate, ATC code: N07XX04; pitolisant, ATC: N07XX11), psychostimulants (modafinil, ATC: N06BA07; methylphenidate, ATC: N06BA04; dextroamphetamine, ATC: N06BA02), tricyclic antidepressants (amitriptyline, ATC: N06AA09; imipramine, ATC: N06AA02; desipramine, ATC: N06AA01; doxepine, ATC: N06AA12; clomipramine, ATC: N06AA04; nortriptyline, ATC: N06AA10; opipramol, ATC: N06AA05; trimipramine, ATC: N06AA06), other antidepressants (fluoxetine, ATC: N06AB03; citalopram, ATC: N06AB04; venlafaxine, ATC: N06AX16; reboxetine, ATC: N06AX18; non-selective MAO inhibitors, ATC: N06AF*), benzodiazepines (ATC: N05BA*), and other agents (selegiline, ATC: N04BD01; ephedrine, ATC: R03CA02; lithium, ATC: N05AN01).
Prevalence estimation and patient characterization (all samples)The age- and sex-standardized point prevalence was calculated for the most recent available date (June 30, 2018) using sample 1. As a denominator, the entire AOK PLUS population continuously insured from July 1, 2013 to June 30, 2018, was used. To consider the impact of the respective definition of the selection criteria on the prevalence estimate, additional scenarios related to the entire period of continuous insurance and the inclusion of outpatient diagnoses documented by any type of physician, including GPs, were tested during sensitivity analysis. The cumulative incidence was evaluated for the period between July 1, 2017, and June 30, 2018. Standardization for the prevalence and incidence estimation calculations was based on the age and gender distribution within the German population (German Federal Statistical Office).
The patient characteristics were descriptively analyzed for the considered patient samples, based on the respective index date or 12 months preceding the index date. In addition to age, sex, and comorbidities based on the Charlson Comorbidity Index (CCI) [20,21] (Supplementary Table 1 in the online-only Data Supplement), we reported whether the patients were pensioners, whether they required care, and the ten most frequently observed comorbidities. Frequency analysis of all the categorical variables was performed, and the number and percentage of patients in each category were reported. The summary statistics, including mean and standard deviation (SD), were reported for the continuous variables.
Diagnostic procedures in incident patients (sample 2)The proportion of the incident patients undergoing any of the predefined diagnostic procedures was analyzed during the 12 months before and after the index date. Polysomnography, maintenance of wakefulness tests (MWTs), multiple sleep latency tests (MSLTs), human leukocyte antigen (HLA) typing, and cerebrospinal fluid analysis were defined as narcolepsy-related diagnostic measures. They were identified in the database using operation and procedure codes (Operationen- und Prozedurenschlüssel, OPS) for inpatient measures and fee schedule position codes (Gebührenordnungsposition, GOP) for outpatient procedures (for corresponding codes, refer to Supplementary Table 2 [in the online-only Data Supplement]).
Cross-sectional analysis: real-world treatment of prevalent patients (sample 1)The pharmacological treatments were descriptively analyzed in a cross-sectional manner for the most recent 12 months (July 1, 2017 to June 30, 2018) for prevalent patients (sample 1) and prevalent patients diagnosed by a GP only (sample 1-sensitivity analysis). The proportion of patients receiving a certain medicinal agent has been reported. Subsequently, the sample was divided into patients who did and did not receive medication during the observational period. These groups were compared based on the main characteristics at the index date and the number of narcolepsy diagnoses during the 12 months preceding the index date, physician visits, hospitalizations, and emergency admissions during the follow-up (mean as well as the number of patients experiencing an event).
Longitudinal analysis: frequency and duration of first-line pharmacological treatment (sample 3)The data of the treatment-naïve patients in sample 3 were analyzed for prescriptions with the above agents longitudinally, starting with the first prescription of a narcolepsy-related agent. The frequency of the prescribed first-line agents was assessed, and Kaplan-Meier analyses were used to evaluate the duration of the first-line therapies. The discontinuation of treatment was assumed if 1) a new agent, which was different from the index agent, was prescribed or 2) there was a supply gap of more than 90 days, with supply being assessed based on the DDDs of the dispensed drugs. The durations of hospitalization were treated as “covered” days, and stockpiling (no disposal of “leftover medication” when submitting a new prescription before the old one was finished) was assumed.
Since several antidepressants were included in the base definition of narcolepsy-related medications for sample selection, the patients who received an antidepressant during the 12 months preceding the index date (which may have been prescribed to treat a psychiatric condition) were not considered treatment-naïve. To account for any uncertainty resulting from this assumption, a sensitivity analysis with a more restrictive definition of “narcolepsy medication” was performed. Here, MAO inhibitors (ATC code: N06AF*) and several antidepressants (amitriptyline, ATC: N06AA09; imipramine, ATC: N06AA02; desipramine, ATC: N06AA01; doxepine, ATC: N06AA12; nortriptyline, ATC: N06AA10; opipramol, ATC: N06AA05; trimipramine, ATC: N06AA06) were not considered as narcolepsy-related. Consequently, during this analysis, the initiation of therapy with these agents was not interpreted to be the beginning of narcolepsy therapy; on the other hand, patients who started therapy with other narcolepsy medications but received one of these agents during the baseline period were considered treatment-naïve with narcolepsy drugs.
Cross-sectional analysis: incremental HCRU and costs (sample 1)HCRU and costs were calculated cross-sectionally for the most recent 12 months for which data was available (July 1, 2017 to June 30, 2018) based on sample 1. To thoroughly account for narcolepsy-associated utilization, an incremental HCRU/cost analysis was conducted, and the prevalent patients were compared with a healthy matched control group. The control group included continuously insured persons alive on July 1, 2017 (index date) who had no diagnosis of a sleeping disorder (ICD-10-GM code: G47.-) during the entire study period. Propensity score matching (PSM) (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8246231/) with the nearestneighbor matching algorithm (caliper <0.001, without replacement) was used to match the two groups. The variables considered in the estimation of the propensity score (assessed based on the 12 months preceding the index date) were age, sex, and CCI.
The HCRU was assessed based on the number of outpatient visits (specialists and GPs, approximated by counted dates of invoiced codes according to the uniform valuation scheme [Einheitlicher Bewertungsmaßstab, EBM]), number of inpatient visits and the number of days in the hospital, number of prescriptions of distinct medications, number of prescriptions of remedies and aids, number of days of rehabilitation, and number of days absent from work. The costs were calculated separately for outpatient care, inpatient care, outpatient medications, remedies and aids, and rehabilitation care.
The reimbursement of services in the outpatient care setting in Germany is regulated by EBM; thus, the services are not invoiced directly based on monetary value but by a system of weighted points. To assess the costs of outpatient care, the weighted points were multiplied by a uniform orientation value, which is defined by the National Association of Statutory Health Insurance Physicians. The inpatient costs covering all performed services and administered drugs during inpatient stays were calculated according to the Diagnosis Related Groups system. The medication costs were calculated based on the outpatient drug prescriptions and pharmacy selling prices relevant at the respective prescription times.
Incremental narcolepsy-related HCRU and costs were calculated as the mean difference between those of the matched prevalent narcolepsy patients and the control group. Significant differences between the two groups were determined using an unpaired t-test or a suitable non-parametric test.
Regulatory aspects and general considerationsAs the study addressed a retrospective anonymized dataset, no ethical review and informed consent of patients were needed. However, the study protocol was reviewed by a scientific steering committee and a data owner. Work on the dataset conformed to all social security data protection requirements.
The statistical analyses were performed using STATA/MP 14 (StataCorp LLC College Station, TX, USA) and Microsoft SQL Server 2014 (Microsoft, Redmond, WA, USA).
RESULTSSelected patientsThe database search identified 1,352 persons with at least one documented narcolepsy diagnosis. At least two confirmed outpatient diagnoses in two separate quarters and/or one inpatient diagnosis were identified for 803 individuals, of which 330 were continuously insured throughout the study period. Of these, 180 were diagnosed by a specialist, of which 133 were alive and prevalent on July 1, 2017 (sample 1). In addition, there were 121 patients diagnosed by GPs only (sample 1-sensitivity).
Seventy-one incident patients (sample 2) received their first narcolepsy diagnosis between July 1, 2014, and June 30, 2017, and 41 treatment-naïve patients (sample 3) started their first narcolepsy therapy between July 1, 2014, and June 30, 2017 (sensitivity sample excluding specific outlined agents: 75 patients). A detailed attrition chart is presented in Fig. 1.
Prevalence estimation and patient characteristicsThe age- and sex-standardized prevalence of narcolepsy in the German population on June 30, 2018, were 5.3 cases per 100,000 persons when only considering patients diagnosed by specialists and 9.1/100,000 when including patients diagnosed by GPs. In another sensitivity analysis, only patients diagnosed within the most recent 12 months were observed, leading to the inclusion of more continuously insured persons as well as a shorter period for fulfilling the diagnosis inclusion criteria. This resulted in a prevalence of 3.1/100,000 people. Overall, a prevalence of 3.1–9.1/100,000 persons can be estimated for Germany. The cumulative incidence evaluated for the period from July 1, 2017 to June 30, 2018, was 0.83 cases per 100,000 persons.
The mean age of the 133 narcolepsy-prevalent patients in sample 1 was 46.2 years (SD: 18.6), with 36.3% being female and 28.6% being retired (Table 2). The mean CCI was 1.2 (SD: 2.1). The most frequently documented diagnoses other than the components of the CCI were essential hypertension (30.8%), depressive disorders (26.3%), and dorsalgia (23.3%).
Patients in incident sample 2 (n=71) and treatment-naïve sample 3 (n=41) had slightly different mean ages (50.9 and 44.5 years, SD: 19.5 and 19.6 years), and they were more frequently female (42.5% and 51.2%) compared with the prevalent sample 1. Somatoform disorders (25.4%), besides hypertension, depressive disorder, and dorsalgia, were common in incident patients, and obesity (19.5%) was among the most frequent diagnoses in the treatment-naïve sample.
Narcolepsy-prevalent patients diagnosed by a GP only (sample 1-sensitivity) were older than those in sample 1 by an average of 17.5 years (Table 2). Consequently, this population had a higher proportion of retired patients (63.3%) and care-dependent persons (20.7%). In addition, the proportion of female patients (50.4%) was higher. After hypertension (67.0%), dorsalgia (51.2%), and lipoprotein metabolism disorder (51.2%) were the most frequently diagnosed comorbidities in this group. Comorbidities were more common in this sample, which was also demonstrated by a higher CCI (2.85, SD: 2.9). Depressive disorders were not found among the ten most frequently diagnosed comorbidities in these patients, whereas it was among the top three in all other subsamples.
Diagnostic procedures in incident patients (sample 2)Among the 71 patients with an observed incident narcolepsy diagnosis, 44 (62.0%) had a claim for at least one of the predefined diagnostic tests during the 24 months around the date of their first diagnosis. The largest proportion of patients completed the MWT (46.5%), followed by MSLT (25.4%) and polysomnography (21.1%) (Supplementary Table 3 in the online-only Data Supplement). Cerebrospinal fluid analysis was performed for three patients (4.2%), and no patient was tested for HLA markers. Generally, the claims for the above procedures were observed with similar frequency during the 12 month pre- and post-index periods. The MWT and MSLT were more commonly documented in the outpatient setting, while polysomnography was more frequently performed in the inpatient setting. Of the patients who underwent at least one diagnostic measure, 27 (75.0%) also completed at least one additional diagnostic procedure, except for patients undergoing a test in the inpatient setting, of whom seven patients (31.8%) completed an additional test. Twenty-seven patients (38.0%) did not undergo any predefined diagnostic tests 12 months before or after the incident narcolepsy diagnosis.
Cross-sectional analysis: real-world treatment of prevalent patients (sample 1)Among the medications used to treat narcolepsy in prevalent patients between July 1, 2017, and June 30, 2018, the most frequently prescribed agent was modafinil (24.1% of observed patients with at least one prescription), followed by sodium oxybate (9.0%), venlafaxine (6.8%), methylphenidate (5.3%), and clomipramine (4.5%) (Fig. 2). Seventy-nine of the 133 patients (59.4%) did not receive any narcolepsy-related medication. Generally, the frequencies of the prescribed agents were lower for the prevalent patients who were diagnosed/treated with GPs only (sample 1-sensitivity).
To investigate whether any agents were disregarded in the base definition of narcolepsy, the 20 most frequently prescribed ATC groups were investigated and reviewed by experts based on their relevance in treating narcolepsy. None of these agents were observed to be relevant to the treatment of narcolepsy.
Supplementary Table 4 (in the online-only Data Supplement) shows the characteristics of the prevalent patients (sample 1) treated with narcolepsy-specific medication compared with those who did not receive any pharmacological therapy during the follow-up. At the index, the untreated sample included slightly fewer females (35.4% vs. 42.6%), fewer pensioners (26.6% vs. 31.5%), and more care-receivers (5.3% vs. 3.7%). The mean age was approximately 46 years, and the mean CCI was 0.91 in both groups. Less than half of the untreated patients (43.0%) were diagnosed with narcolepsy during the pre-index period, compared to 89.0% in the treated group. There were also differences related to medical consultations during the follow-up: GPs/neurologists in association with a narcolepsy claim were consulted by 64.8% and 72.2% of the patients receiving treatment versus 6.3%/10.1% of the untreated patients, resulting in a difference in the mean number of consultations per patient-year (Supplementary Table 4 in the online-only Data Supplement).
Longitudinal analysis: frequency and duration of first-line pharmacological treatment (sample 3)Among the 41 treatment-naïve patients in sample 3, the most frequently prescribed first-line agent was modafinil (19 patients, 46.3%) (Fig. 3). Only four patients received the second most prescribed first-line drug, which was benzodiazepine lorazepam (9.8%), followed by citalopram, venlafaxine, sodium oxybate, pitolisant, and diazepam; each was prescribed to two patients (4.9%). A similar distribution was found in the sensitivity sample, which contained treatment-naïve patients based on a more stringent definition of narcolepsy-related medications related to their pre-index period, in which a higher proportion of patients received the antidepressant citalopram (13.3%), and four patients received benzodiazepine medazepam (5.3%).
Regarding the treatment duration for the first-line agents, the median time to treatment discontinuation was 73 days for treatment-naïve patients in sample 3 and 97.5 days for patients in the sensitivity sample. Kaplan-Meier curves showing the persistence of the first-line therapy are shown in Supplementary Fig. 1 (in the online-only Data Supplement).
Cross-sectional analysis: incremental HCRU and costsFor the selection of the healthy control group, we identified 1.86 million continuously insured persons in the database who had no claim of any sleeping disorder during the time of observation. The mean age and proportion of females in this group were higher than those in the narcolepsy-prevalent sample. Using PSM, 133 controls were matched to the narcolepsy-prevalent sample 1. No significant differences in age, sex, and CCI were observed between the matched groups (Supplementary Table 5 in the online-only Data Supplement).
During the follow-up between July 1, 2017, and June 30, 2018, patients in the narcolepsy group visited a GP 3.3 times per year, consulted a specialist 8.2 times, and were hospitalized 0.5 times on average (Table 3). A comparison with the matched control group showed that narcolepsy-prevalent patients consulted physicians significantly more often (GP visits: 0.41, 95% confidence interval [CI]: 0.04–0.86, p=0.005; specialist visits: 2.86, 95% CI: 1.38–4.33, p<0.001) and were hospitalized significantly more often (0.23, 95% CI: 0.03–0.43, p=0.023). No significant difference between the groups was found for the other investigated categories, with borderline significance for the number of days in the hospital (2.13, 95% CI: -0.31–4.57, p=0.087) (Table 3).
For the narcolepsy-prevalent sample, the mean total healthcare cost was €4,654 (SD: €8,811); the cost of medications (€1,988, SD: €7,011), inpatient care (€1,261, SD: €4,707), and outpatient care (€1,006, SD: €753) were the three main drivers (Table 4). Differences in healthcare costs between the narcolepsy- prevalent sample and the matched control group were significant for outpatient care (€312, 95% CI: 146–478, p<0.001) and medications (€1,588, 95% CI: 379–2,797, p=0.010). Overall, additional costs due to narcolepsy of €2,429 per year (95% CI: 748–4,110, p=0.005) were observed.
DISCUSSIONThe objective of our study was to generate real-world data on the epidemiology and treatment of patients with narcolepsy in Germany.
In our study, the age- and sex-standardized prevalence of narcolepsy within the German population was between 3.1 and 9.1 cases per 100,000 persons, depending on patient inclusion criteria and the length of the observational period. The cumulative incidence was 0.83% per 100,000 persons between July 1, 2017, and June 30, 2018. While another study reported a similar incidence (0.64/100,000) in the overall German population in 2011 [22], our estimated prevalence was lower than most of those reported in the existing literature. The prevalence of narcolepsy (defined as NT1 and NT2) has been estimated between 37 and 80 per 100,000 in studies conducted in the USA [23-25], and 15–34 per 100,000 in studies involving populations in Korea and Hong Kong [26,27]. An investigation of the Finnish twin cohort reported a prevalence of 26 per 100,000 [28], while a study by Ohayoy et al. [29] found that 47 per 100,000 persons in the general European population experience the condition. One recent study conducted in the Spanish region of Catalonia reported a prevalence similar to the one calculated in our analysis (5.2/100,000) [30].
The above differences generally indicate a lower narcolepsy prevalence in Germany, but they may also be due to our methodology, which required at least one inpatient diagnosis or two confirmed outpatient diagnoses of a specialist for inclusion in the analyses. We followed a more conservative approach because we aimed to avoid the inclusion of patients with misdiagnoses or incorrect documentation. Other studies with less stringent approaches may have included patients with unconfirmed diagnoses or diagnoses of other sleep disorders. Furthermore, the actual prevalence of narcolepsy in Germany may be higher than we have reported because patients with narcolepsy experience an average delay in diagnosis of 6–15 years or are never correctly diagnosed with narcolepsy. Health insurance data generally only report patients who claim health care services and are diagnosed. Furthermore, several patients are misdiagnosed with other conditions due to the ambiguous definition of NT2 conditions [14,31-33]. We also identified a substantial number of patients with other sleep disorders [33], specifically with sleep apnea [34]. In addition, cataplexy may be erroneously identified as epilepsy in some patients, leading to delayed diagnosis or misdiagnosis [3].
In addition to the main prevalent sample in our study consisting of patients diagnosed by a specialist, we identified a considerable number of individuals diagnosed by GPs only (without having any diagnosis of narcolepsy documented by a specialist or hospital). These patients were more likely to be female (50.4% vs. 36.3%) and significantly older than those diagnosed by specialists (mean age, 63.7 years vs. 46.2 years). Consequently, comorbidities were more common in the sample.
The most frequently observed comorbidities among patients with narcolepsy are dorsalgia, hypertension, depression, somatoform disorders, and obesity. Sleep apnea, depression, and disorders of excessive somnolence were the most common differential diagnoses. This is consistent with reports in existing literature that psychiatric conditions/mental illness (particularly depression) [11,35,36], cardiovascular diseases [11,13], back pain [11,37], obesity [11,37], and sleep disorders, such as sleep apnea and restless leg syndrome [11,14,37,38], are significantly more common in narcolepsy patients than in control groups.
Regarding the diagnostic procedures used to identify narcolepsy, only approximately half of the incident patient population underwent an MWT during the 24 months around their
incident diagnosis, while only a quarter completed an MSLT, and 21% underwent polysomnography. These are remarkably low numbers, as polysomnography [33,34] and MSLT39 are standard procedures for the assessment of narcolepsy and are described in the treatment guidelines to distinguish narcolepsy from idiopathic hypersomnia and other sleep disorders [40]. Interestingly, the observed patients either had claims for several tests or did not receive any narcolepsy-related diagnostic tests at all, while 38% of the patients had no claims for a test at all; roughly three-quarters of those patients who underwent a diagnostic procedure also completed a second one during the 24 months around their incident narcolepsy diagnosis. This may suggest the heterogeneity of the quality of medical care across the patient groups and may also be related to challenges in accessing the right diagnostic tests in clinical practice. However, these results may also be due to budgetary restrictions, where not all diagnostic measures are claimable, at least within certain healthcare sectors.
In our analysis, the majority of patients receiving narcolepsyrelated pharmacological therapy were treated with modafinil, which is a recommended first-line medication option for NT2 in Germany [17]. Several patients also received sodium oxybate, venlafaxine, methylphenidate, clomipramine, and other antidepressants. While methylphenidate is recommended as second- line therapy for NT2, sodium oxybate and clomipramine are labeled agents for NT1 narcolepsy, and venlafaxine is used as an off-label medication for NT1 and comorbid depression [17,18]. Only one patient received pitolisant, potentially due to the fairly recent approval of the agent in Germany in 2016. More than half of the prevalent study sample 1 did not receive any pharmacological therapy, even though it is commonly agreed that most patients need lifelong medicinal treatment to manage their symptoms [1]. The exact reasons for the observed undertreatment are unknown and need to be further explored. This may be attributed to the reluctance of physicians prescribing the respective agents according to patient wishes or efficacy and tolerability profiles. Furthermore, even if the number of newly treated patients (sample 3) in the longitudinal analysis were quite limited, it needs to be highlighted that a high proportion of patients discontinued the first-line treatment within the first quarter after treatment initiation (median time to treatment discontinuation: 73 days). Therefore, a substantial proportion of untreated patients in sample 1 may have received their respective treatment during previous years but discontinued it. In our sample, the majority of patients without treatment during the observational period did not receive any narcolepsy-specific treatment during the 12 months preceding the index date.
In our incremental approach, narcolepsy-prevalent patients utilized significantly more healthcare resources compared with matched controls in relation to outpatient consultations and hospitalizations. This is consistent with previous studies assessing narcolepsy-related HCRU in Denmark [41,42] and the US [7,43,44]. Some of these studies also reported medication use [42,44], inpatient days [43], and emergency admission [7,43,44] to be significantly increased in patients with narcolepsy. The mean total direct healthcare-related costs in our study were €4,654 per year for patients with narcolepsy, which is almost twice as much as that for the matched control group. Our results are similar to the findings of a Danish study that reported direct healthcare costs of narcolepsy to be €4,163/PY with a €2,813 excess compared with the control group [41] and a German survey that found direct costs of €3,180/PY [45]. Our findings are lower than those of another Danish study42 (€9,572 total cost) and several retrospective studies conducted in the USA ($15,797 total cost with $13,348 excess [43]; $22,828 for outpatient care with $12,667 excess [7]; $27,642 for hospitalizations with $10,998 excess [7]; $8,346 for medical services with $4,199 excess [44]; $3,356 for medication with $2,242 excess [44]). It should be noted that the above studies were conducted in different healthcare settings and used different methodologies.
LimitationsGenerally, the nature of the claims dataset used as a basis for this analysis precludes selection bias and facilitates generalizability; however, some limitations should be acknowledged. The main limitation of our study is the limited sample size, which affects the generalizability of our results. The analyses of the real-world treatment were especially aggravated, as no meaningful results could be reported on treatment journeys and combination therapies. Second, because of the nature of the analyzed data, it was not possible to differentiate between narcolepsy subtypes NT1 and NT2, as the ICD10-GM code G47.4 refers to conditions with and without cataplexy. Third, even though the incident sample only included patients who had not been diagnosed with narcolepsy during the 12-month pre-index period, we cannot exclude the possibility that these patients had received a respective diagnosis before the observational period. Fourth, there is limited socio-economic information available in the claims data of German sickness funds. Thus, information about social status, living circumstances, health-related quality of life, or behavioral patterns could not be considered in our study. It should also be noted that several of the agents considered narcolepsy-related medications are prescribed for off-label conditions and may have been used to treat other indications in some of the patients. From the information documented in the claims data, it is not possible to infer the condition for which an agent was prescribed. Antidepressants and benzodiazepines are agents commonly prescribed to patients experiencing psychiatric disorders, which are among the most frequently diagnosed comorbidities of narcolepsy [35,36]. Finally, using only data from one regional German sickness fund, the results may not be fully representative of the entire German population, although we mitigated this bias by estimating age- and sex-standardized prevalence and incidence.
ConclusionNarcolepsy is a rare disease, and information on the epidemiology, diagnosis, treatment, and burden of disease is limited, especially in Germany. This analysis has provided initial insights, with a further need for systematic research on realworld treatments, especially the reasons for not treating narcolepsy patients with pharmacologic therapies.
Our results showed a high burden of comorbidities in patients with narcolepsy, which may be associated with the increased complexity of treatment. The disease results in significantly higher utilization of healthcare resources, especially in outpatient care.
With a considerable proportion of patients not receiving appropriate diagnostic measures as well as a substantial proportion of patients without pharmacological treatment, our study highlights the unmet need for the diagnosis and treatment of narcolepsy patients in Germany.
Supplementary MaterialsThe online-only Data Supplement is available with this article at https://doi.org/10.13078/jsm.210007.
Supplementary Table 2.Identification of diagnostic procedures via OPS and GOP codes Supplementary Table 3.Diagnostic procedures taken by incident patients Supplementary Table 4.Characteristics and resource utilization of treated and untreated prevalent patients (sample 1) Supplementary Table 5.Patient characteristics before and after propensity score matching Supplementary Figure 1.Persistence to first-line narcolepsy therapy in treatment-naïve patients (sample 3). Kaplan-Meier curves showing the proportion of patients without changes or treatment discontinuation over time for the treatment-naïve sample (A) and the treatmentnaïve sensitivity sample (B) with the altered definition of narcolepsy-specific medication, excluding certain monoamine oxidase inhibitors and certain antidepressants as described in the methods section. CI, confidence interval. Notes
Conflicts of Interest
Sabrina Müller participated in this study as a staff member of IngressHealth; the work of Ingress-Health in this study was sponsored by Takeda Pharma Vertrieb GmbH & Co. KG.
Alina Brandes was an employee of Takeda Pharma Vertrieb GmbH & Co. KG at the time of the study conduct.
Julian Knierim and Milan Novakovic are employees of Takeda Pharma Vertrieb GmbH & Co. KG.
Thomas Wilke has received honoraria from several pharmaceutical/consultancy companies, e.g., Bayer, GSK, UCB, Takeda, Boehringer Ingelheim, and Janssen.
Ulf Maywald does not have any conflicts of interest, except those potentially related to his employer, AOK PLUS.
Ingo Fietze has received consultancy fees and grants from Takeda, Jazz, Bayer, and Idorsia.
Author Contributions
Conceptualization: Sabrina Müeller, Alina Brandes, Julia Knierim, Thomas Wilke, Ulf Maywald. Formal analysis: Sabrina Müeller. Methodology: Thomas Wilke. Project administration: Alina Brandes, Julia Knierim. Supervision: Milan Novakovic, Ulf Maywald, Ingo Fietze. Validation: Ulf Maywald, Ingo Fietze. Writing—original draft: Sabrina Müeller, Thomas Wilke. Writing—review & editing: Alina Brandes, Julia Knierim, Milan Novakovic, Ulf Maywald, Ingo Fietze.
Fig. 1.Patient inclusion criteria and selected patient subsamples. *including neurology/psychotherapy/psychiatry/neuropediatrics/pediatric psychiatry and psychotherapy/pulmonology/pediatrics (AGS codes 51, 53, 58, 60, 61, 44, 47, 30, 45, and 40). AGS, Arztgruppenschlüssel; GP, general practitioner.
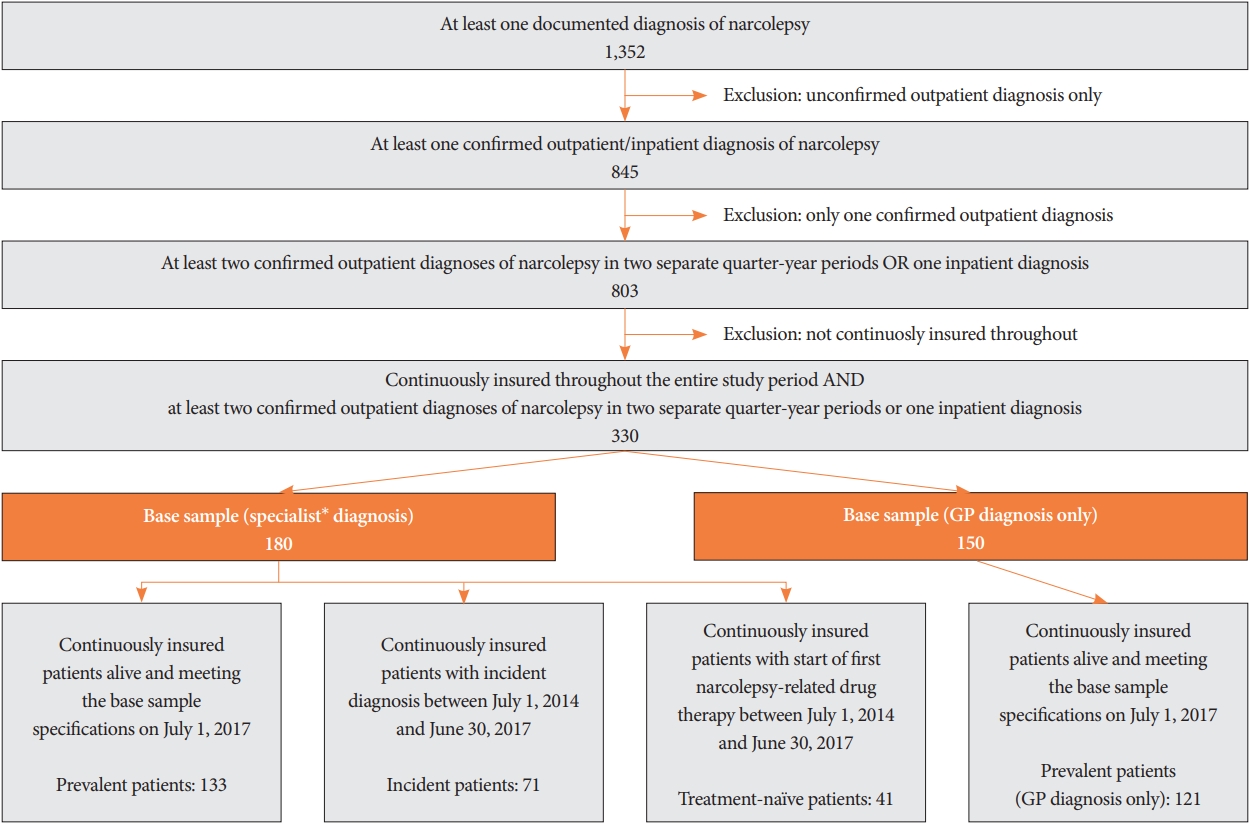
Fig. 2.Description of drug treatments for narcolepsy-prevalent patients. Percentage of patients who received at least one prescription of the respective narcolepsy-related agent between July 1, 2017 and June 30, 2018 are shown for the prevalent sample and for the prevalent sample diagnosed by a GP only. GP, general practitioner. 
Fig. 3. Distribution of first-line agents prescribed to treatment-naïve narcolepsy patients (sample 3). The number and percentage of patients receiving the respective agent as first-line therapy are displayed. *sensitivity sample with a more stringent definition of narcolepsy specific medication relating to the pre-index period, excluding certain tricyclic antidepressants and monoamine oxidase inhibitors as defined in the methods section. 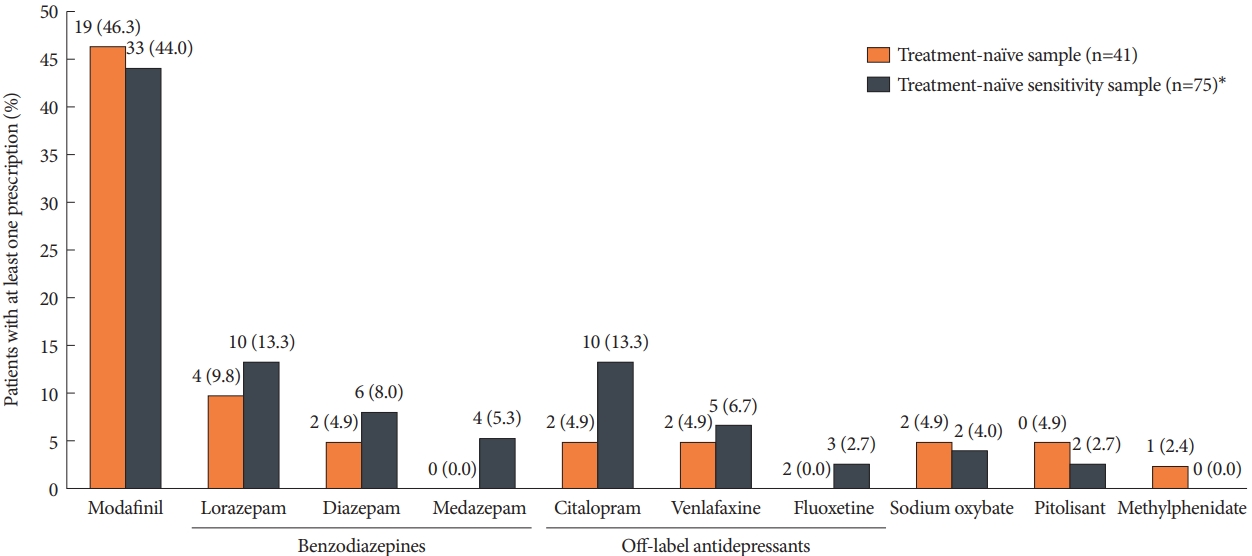
Table 1.Sample definition of patients with narcolepsy enrolled in the study and subsequent analyses
Table 2.Main characteristics of study samples
Table 3.Incremental HCRU of the narcolepsy-prevalent sample compared to the matched control group
Table 4.Healthcare cost of narcolepsy-prevalent sample 1, compared to the matched control group
REFERENCES1. Barateau L, Dauvilliers Y. Recent advances in treatment for narcolepsy. Ther Adv Neurol Disord 2019;12:1756286419875622. https://doi.org/10.1177/1756286419875622.
2. Mahoney CE, Cogswell A, Koralnik IJ, Scammell TE. The neurobiological basis of narcolepsy. Nat Rev Neurosci 2019;20:83-93. https://doi.org/10.1038/s41583-018-0097-x.
4. Krahn LE, Lymp JF, Moore WR, Slocumb N, Silber MH. Characterizing the emotions that trigger cataplexy. J Neuropsychiatry Clin Neurosci 2005;17:45-50. https://doi.org/10.1176/jnp.17.1.45.
5. Thorpy M. International classification of sleep disorders. In: Chokroverty S. Sleep disorders medicine. New York: Springer, 2017;475-484.
6. Mayer G, Kesper K, Peter H, Ploch T, Leinweber T, Peter JH. The implications of gender and age at onset of first symptoms in narcoleptic patients in Germany—results from retrospective evaluation of hospital records. Somnologie 2002;6:13-18. https://doi.org/10.1046/j.1439-054x. 2002.02005.x.
7. Flores NM, Villa KF, Black J, Chervin RD, Witt EA. The humanistic and economic burden of narcolepsy. J Clin Sleep Med 2016;12:401-407. https://doi.org/10.5664/jcsm.5594.
8. Bayard S, Croisier Langenier M; Cochen De Cock V, Scholz S, Dauvilliers Y. Executive control of attention in narcolepsy. PLoS One 2012;7:e33525. https://doi.org/10.1371/journal.pone.0033525.
9. Pizza F, Jaussent I, Lopez R, et al. Car crashes and central disorders of hypersomnolence: a French study. PLoS One 2015;10:e0129386. https://doi.org/10.1371/journal.pone.0129386.
10. Ingravallo F, Vignatelli L, Brini M, et al. Medico-legal assessment of disability in narcolepsy: an interobserver reliability study. J Sleep Res 2008;17:111-119. https://doi.org/10.1111/j.1365-2869.2008.00630.x.
11. Cohen A, Mandrekar J, St Louis EK, Silber MH, Kotagal S. Comorbidities in a community sample of narcolepsy. Sleep Med 2018;43:14-18. https://doi.org/10.1016/j.sleep.2017.11.1125.
12. Morse AM, Sanjeev K. Narcolepsy and psychiatric disorders: comorbidities or shared pathophysiology? Med Sci (Basel) 2018;6:16. https://doi.org/10.3390/medsci6010016.
13. Dauvilliers Y, Jaussent I, Krams B, et al. Non-dipping blood pressure profile in narcolepsy with cataplexy. PLoS One 2012;7:e38977. https://doi.org/10.1371/journal.pone.0038977.
14. Frauscher B, Ehrmann L, Mitterling T, et al. Delayed diagnosis, range of severity, and multiple sleep comorbidities: a clinical and polysomnographic analysis of 100 patients of the innsbruck narcolepsy cohort. J Clin Sleep Med 2013;9:805-812. https://doi.org/10.5664/jcsm.2926.
15. Marín Agudelo HA, Jiménez Correa U, Carlos Sierra J, Pandi-Perumal SR, Schenck CH. Cognitive behavioral treatment for narcolepsy: can it complement pharmacotherapy? 2014;7:30-42. Sleep Sci 2014;7:30-42. https://doi.org/10.1016/j.slsci.2014.07.023.
16. Koziorynska EI, Rodriguez AJ. Narcolepsy: clinical approach to etiology, diagnosis, and treatment. Rev Neurol Dis 2011;8:e97-e106.
17. Gerloff C. Narkolepsie. Diener HC, Weimar C. Leitlinien für Diagnostik und therapie in der neurologie. Stuttgart: Thieme, 2012.
18. Kallweit U, Bassetti CL. Pharmacological management of narcolepsy with and without cataplexy. Expert Opin Pharmacother 2017;18:809-817. https://doi.org/10.1080/14656566.2017.1323877.
19. Thorpy MJ, Dauvilliers Y. Clinical and practical considerations in the pharmacologic management of narcolepsy. Sleep Med 2015;16:9-18. https://doi.org/10.1016/j.sleep.2014.10.002.
20. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245-1251. https://doi.org/10.1016/0895-4356(94)90129-5.
21. Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol 2004;57:1288-1294. https://doi.org/10.1016/j.jclinepi.2004.03.012.
22. Oberle D, Drechsel-Bäuerle U, Schmidtmann I, Mayer G, Keller-Stanislawski B. Incidence of narcolepsy in Germany. Sleep 2015;38:1619-1628. https://doi.org/10.5665/sleep.5060.
23. Longstreth WT Jr, Ton TG, Koepsell T, Gersuk VH, Hendrickson A, Velde S. Prevalence of narcolepsy in King County, Washington, USA. Sleep Med 2009;10:422-426. https://doi.org/10.1016/j.sleep.2008.05.009.
24. Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep 2002;25:197-202. https://doi.org/10.1093/sleep/25.2.197.
25. Scheer D, Schwartz SW, Parr M, Zgibor J, Sanchez-Anguiano A, Rajaram L. Prevalence and incidence of narcolepsy in a US health care claims database, 2008-2010. Sleep 2019;42:zsz091. https://doi.org/10.1093/sleep/zsz091.
26. Shin YK, Yoon IY, Han EK, et al. Prevalence of narcolepsy-cataplexy in Korean adolescents. Acta Neurol Scand 2008;117:273-278. https://doi.org/10.1111/j.1600-0404.2007.00930.x.
27. Wing YK, Li RH, Lam CW, Ho CK, Fong SY, Leung T. The prevalence of narcolepsy among Chinese in Hong Kong. Ann Neurol 2002;51:578-584. https://doi.org/10.1002/ana.10162.
28. Hublin C, Kaprio J, Partinen M, et al. The prevalence of narcolepsy: an epidemiological study of the Finnish Twin Cohort. Ann Neurol 1994;35:709-716. https://doi.org/10.1002/ana.410350612.
29. Ohayon MM, Priest RG, Zulley J, Smirne S, Paiva T. Prevalence of narcolepsy symptomatology and diagnosis in the European general population. Neurology 2002;58:1826-1833. https://doi.org/10.1212/wnl.58.12.1826.
30. Tió E, Gaig C, Giner-Soriano M, et al. The prevalence of narcolepsy in Catalunya (Spain). J Sleep Res 2018;27:e12640. https://doi.org/10.1111/jsr.12640.
31. Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med 2014;15:502-507. https://doi.org/10.1016/j.sleep.2014.01.015.
32. Morrish E, King MA, Smith IE, Shneerson JM. Factors associated with a delay in the diagnosis of narcolepsy. Sleep Med 2004;5:37-41. https://doi.org/10.1016/j.sleep.2003.06.002.
33. Flygare J, Parthasarathy S. Narcolepsy: let the patient’s voice awaken us! Am J Med 2015;128:10-13. https://doi.org/10.1016/j.amjmed.2014.05.037.
34. Rosenberg R, Hirshkowitz M, Rapoport DM, Kryger M. The role of home sleep testing for evaluation of patients with excessive daytime sleepiness: focus on obstructive sleep apnea and narcolepsy. Sleep Med 2019;56:80-89. https://doi.org/10.1016/j.sleep.2019.01.014.
35. Black J, Reaven NL, Funk SE, et al. Medical comorbidity in narcolepsy: findings from the Burden of Narcolepsy Disease (BOND) study. Sleep Med 2017;33:13-18. https://doi.org/10.1016/j.sleep.2016.04.004.
36. Lee MJ, Lee SY, Yuan SS, et al. Comorbidity of narcolepsy and depressive disorders: a nationwide population-based study in Taiwan. Sleep Med 2017;39:95-100. https://doi.org/10.1016/j.sleep.2017.07.022.
37. Jennum P, Ibsen R, Knudsen S, Kjellberg J. Comorbidity and mortality of narcolepsy: a controlled retro- and prospective national study. Sleep 2013;36:835-840. https://doi.org/10.5665/sleep.2706.
38. Plazzi G, Ferri R, Antelmi E, et al. Restless legs syndrome is frequent in narcolepsy with cataplexy patients. Sleep 2010;33:689-694. https://doi.org/10.1093/sleep/33.5.689.
39. Mitler MM, Hajdukovic R, Erman M, Koziol JA. Narcolepsy. J Clin Neurophysiol 1990;7:93-118. https://doi.org/10.1097/00004691-199001000-00008.
40. Wise MS. Objective measures of sleepiness and wakefulness: application to the real world? J Clin Neurophysiol 2006;23:39-49. J Clin Neurophysiol 2006;23:39-49. https://doi.org/10.1097/01.wnp.0000190416.62482.42.
41. Jennum P, Knudsen S, Kjellberg J. The economic consequences of narcolepsy. J Clin Sleep Med 2009;5:240-245.
42. Jennum P, Ibsen R, Petersen ER, Knudsen S, Kjellberg J. Health, social, and economic consequences of narcolepsy: a controlled national study evaluating the societal effect on patients and their partners. Sleep Med 2012;13:1086-1093. https://doi.org/10.1016/j.sleep.2012.06.006.
43. Carls G, Reddy SR, Broder MS, et al. Burden of disease in pediatric narcolepsy: a claims-based analysis of health care utilization, costs, and comorbidities. Sleep Med 2020;66:110-118. https://doi.org/10.1016/j.sleep.2019.08.008.
44. Black J, Reaven NL, Funk SE, et al. The Burden of Narcolepsy Disease (BOND) study: health-care utilization and cost findings. Sleep Med 2014;15:522-529. https://doi.org/10.1016/j.sleep.2014.02.001.
45. Dodel R, Peter H, Walbert T, et al. The socioeconomic impact of narcolepsy. Sleep 2004;27:1123-1128. https://doi.org/10.1093/sleep/27.6.1123.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||