A Ten-Year Follow-Up Case Report on the Treatment of Severe Obstructive Sleep Apnea Using a Mandibular Advancement Device
Article information
Abstract
This report describes the challenging case of a 45-year-old male Caucasian patient with morbid obesity and severe obstructive sleep apnea who was followed up for over 10 years by a multidisciplinary team that included sleep and dental medicine professionals, physiotherapists, and nutritionists. The patient underwent seven polysomnographic studies during the follow-up period; furthermore, nocturnal digital monitoring (NDM) was performed during the COVID-19 pandemic. The polysomnographic study revealed that the apnea-hypopnea index of the patient reduced from 131.7 ev/h to 9.4 ev/h without symptoms, while his NDM oxygen desaturation index varied because of weight gain during the COVID-19 pandemic. This case report highlights the importance of a multidisciplinary team in ensuring the success of mandibular advancement device therapy with excellent clinical outcomes. Furthermore, this report suggests that NDM was a good tool for follow-up during the COVID-19 pandemic.
INTRODUCTION
Patients with obstructive sleep apnea syndrome (OSAS) are often non-adherent to continuous positive airway pressure (CPAP) therapy. Therefore, using a mandibular advancement device (MAD) may be a suitable approach for improving compliance [1], especially in cases of obstructive sleep apnea (OSA) in patients with craniofacial disharmony [2]. Type I polysomnography (PSG) is a mandatory laboratory sleep study [3]. Although patients with OSA are diagnosed and monitored using this test, it is expensive, time-consuming, and challenging to access [4,5]. Moreover, some patients are unwilling to undergo a second PSG study. This challenge is frequently encountered in Dental Sleep Medicine and is a source of concern as monitoring the outcomes of MAD therapy is necessary.
and the reliable Oxistar® sensor (Biologix Sistemas LTDA, São Paulo, SP, Brazil), is a suitable alternative method for assessing the severity of OSA. This integrated approach has diagnostic capabilities that effectively detect and evaluate OSA severity [6]. Studies have shown that despite some limitations in the diagnosis of OSA [4], NDM was an option for patient monitoring during the COVID-19 pandemic when access to diagnostic services was restricted. Only a few published randomized studies investigated the management of severe OSAS using MAD therapy. Herein, we present a challenging case of a patient with OSAS who was referred by a sleep physician to undergo customized MAD therapy [7].
CASE REPORT
The patient was a 45-year-old morbidly obese (body mass index=40 kg/m²) male Caucasian. He initially underwent two all-night polysomnographic studies in a sleep laboratory comprising basal and CPAP titration in December 2010 and May 2011, respectively (Table 1). Using diagnostic PSG findings, he was diagnosed with severe OSAS with an apnea-hypopnea index (AHI) 131.7 ev/h. A standardized sleep laboratory titration trial revealed that the optimal titration pressure was 6 cmH2O with a remaining AHI of 4.8 ev/h. However, the patient could not tolerate CPAP therapy as he was claustrophobic; therefore, he was nonadherent to treatment. Owing to low water pressure and phenotypic characteristics, the sleep physician prescribed a MAD.

Type I and IV polysomnography test: basal, with CPAP titration and Diors® mandibular advancement device adjustment
PSG was performed using Brain Wave II PSG Neuro Virtual (Neurovirtual Produtos Medicos LTDA, Barueri, SP, Brazil) by physicians who were specially trained in sleep medicine per the American Academy of Sleep Medicine (AASM) manual for the scoring of sleep with 29 channels. The channels consisted of referential alternating current (AC) inputs (8 electroencephalographic, 2 electrooculograms, and 3 auxiliary), bipolar AC inputs (1 electromyogram, 1 electrocardiogram, 1 snore, 1 flow, 1 pressure, oximetry, 2 efforts, 1 position, 1 leg movement, and 2 auxiliary), and 3 direct current inputs. The sleep stages of the patient (wake [W], sleep stage 1 [N1], sleep stage 2 [N2], sleep stage 3 [N3], and sleep stage rapid eye movement [R]) were scored according to the AASM scoring rules. AHI was defined as the number of apnea plus hypopnea episodes per hour of sleep, and OSA was defined as an AHI ≥5 ev/h.
The clinical history of the patient was considerable for excessive daytime sleepiness (ESS=14), non-restorative sleep, respiratory allergy with difficult nasal breathing and intermittent snoring during sleep, and a sedentary lifestyle. The patient also had a history of prediabetes, hypertension, gastroesophageal reflux disease, nasal polyps, and septal deviation; he was also on medications for type 2 diabetes mellitus and hypertension, which were well controlled.
The neck and waist circumferences of the patient were 46 cm and 130 cm, respectively. Further physical examination revealed that the patient had retrognathia, macroglossia with a scalloped tongue, and an oropharynx with Mallampati and Brodsky classifications IV and 2, respectively. The patient had good oral health with no signs suggestive of crepitus in the temporomandibular joint. The patient presented with an acceptable mandibular protrusion and neuromuscular response and had a favorable dental prognosis.
Two clinical factors were critical to the decision-making process during the management of this challenging case. The patient showed good neuromuscular responses to stimuli from the pharyngeal muscles despite having a flaccid soft palate with an enlarged uvula, voluminous tongue, and good mandibular advancement dynamic. The neuromuscular response was assessed by instructing the patient to repeatedly pronounce the letter “/a:/” while observing the movement of the uvula to assess for improvements in the modified Mallampati score. In addition, a considerable reduction in the AHI was observed with lower CPAP titration pressure. These factors predicted a positive prognosis for MAD adaptation. The patient was informed about the treatment, which included septal deviation surgery and weight loss. The low titration pressure of CPAP indicated that MAD therapy was likely to have good outcomes, despite the obesity and severity of the AHI presented by the patient, which were not indicators of a good prognosis in this case.
Diors® MAD was selected for the treatment. The Diors® MAD design (Fig. 1) uses knowledge of functional possibilities to stimulate tongue protrusion, lip sealing, and nasal breathing. Despite these difficulties, the patient was motivated to adhere to this therapeutic option. A multidisciplinary team approach was recommended to optimize the response to the Diors® MAD. The patient had nasal polyps and septal deviation; therefore, he was referred to an otorhinolaryngologist for a septoplasty to increase nasal patency. The patient complained of transitory bilateral temporomandibular discomfort during MAD therapy; however, this was resolved through physiotherapy. In addition, the patient received nutritional therapy and personal training to reduce weight and maintain good sleep hygiene.
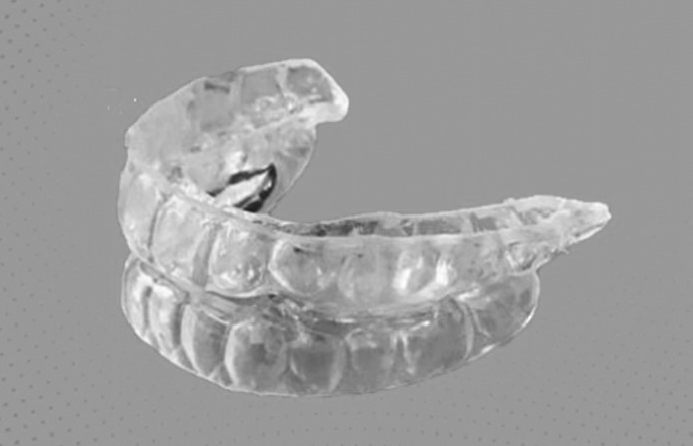
Illustration of the Diors® (LFB & Associados LTDA., Jundiaí, São Paulo, Brazil) mandibular advancement device used in the patient.
The results of seven type I and IV PSG studies are shown in Table 1. Importantly, six of the type I PSG studies were administered at the same sleep laboratory, while six of the type IV PSG studies were performed out-of-center. Only the last type I and IV PSG studies were performed in another sleep laboratory. The PSG study results showed improvements in respiratory parameters and sleep efficiency. The latest type I and IV PSG study results showed some differences in the total sleep time, probably due to the beginning of the recording; however, in the recorded sleep time, the respiratory events were similar with AHI of 9.4 (type I PSG) and oxygen desaturation index (ODI) of 9.5 (type IV PSG).
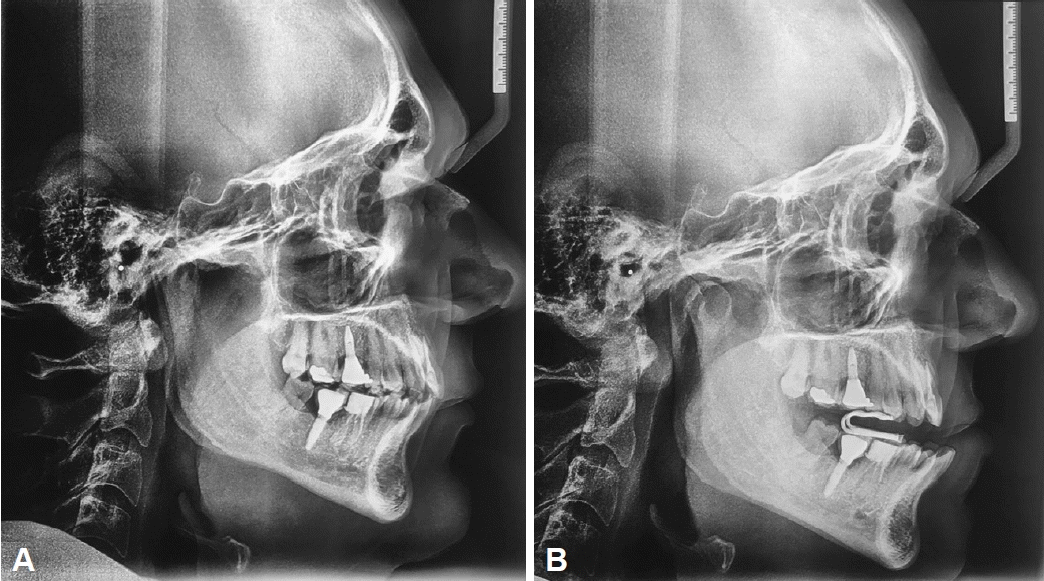
In two teleradiographs analyses without and with the Diors® MAD (Fig. 2), we demonstrated the mode of action of MAD therapy in pharyngeal airspace improvement and hyoid distance reduction to the mandibular plane. In an observational study of 33 patients with OSA treated with Diors® MAD [8], the authors have associated this mode of action with a considerable reduction in AHI.
DISCUSSION
Although several studies that have compared CPAP with MAD have shown that CPAP is more effective in reducing AHI [5], other studies have shown no clinically relevant difference between CPAP and MAD in patients with mild-to-moderate OSAS when both treatment modalities are titrated or advanced objectively [7-9]. Despite the greater efficacy of CPAP in reducing AHI, in comparative trials of equivalence of health outcomes with CPAP and MAD, even in severe OSAS, MAD has been shown to be effective in treating OSA, not only by improving AHI but also by improving a variety of physiological and behavioral-cognitive outcomes [10].
During the COVID-19 pandemic, PSG laboratories became more difficult to access; therefore, type IV PSG with Biologix NDM was proposed, as this was found to have diagnostic value in detecting OSA. Despite being a simplified PSG option, by evaluating the ODI, snoring, heart rate, and body movements, we could determine that in this patient, type IV PSG with Biologix NDM for Dental Sleep Medicine practice was a very useful complementary tool during the COVID-19 pandemic. However, despite the similarity between the ODI and AHI, we cannot replace the tests. We agree that type IV PSG with Biologix NDM is a complementary tool, and conventional PSG is necessary for a complete follow-up.
Craniofacial phenotypic characteristics are important risk factors in patients with OSAS and have already been highlighted in the literature [2]. Knowing how to identify these phenotypic characteristics is important for predicting the success of OSA therapy. Although the patient did not lose enough weight, the Diors® MAD reduced AHI by nearly 90%, and improved ESS scores and fatigue symptoms. The patient reported consistent and excellent adherence to the MAD therapy, consistently using it every night. He also felt well-adapted and satisfied with his quality of sleep and overall life.
This challenging case showed that MAD could be used to treat a patient with severe OSAS with craniofacial phenotypic characteristics and non-adherence to CPAP; therefore, this therapy can be considered as an option for such patients. However, a multidisciplinary team is important to assist in the adaptation and adherence to MAD therapy. Furthermore, monitoring of OSAS in Dental Sleep Medicine practice in this patient was a useful tool for therapy follow-up during the COVID-19 pandemic.
Notes
Availability of Data and Material
Data sharing is not applicable to this article as no datasets were generated or analyzed during the study.
Ethics Statement
This clinical case was approved by the ethics committee of the Dental School of Piracicaba, University of Campinas School of Medical Sciences (UNICAMP), SP, Brazil (CAAE:61347821.0.0000.5418). The patient was informed of the study and provided consent for treatment. In addition, this case report was prepared in line with the CARE guidelines.
Diors® is registered and patented in the International Institute of Industrial Property (INPI) by the first author.
The first author developed the mandibular advancement device (Diors®) and holds trademarks and patents at the INPI. In addition, the type IV PSG Biologix was borrowed during the COVID-19 pandemic for two studies (this case report and another observational study that is currently being developed). These does not affected the publication of this manuscript.
Author Contributions
Conceptualization: Denise Fernandes Barbosa, Almiro José Machado Júnior. Data curation: Denise Fernandes Barbosa. Formal analysis: Denise Fernandes Barbosa, Almiro José Machado Júnior. Investigation: Denise Fernandes Barbosa. Methodology: Denise Fernandes Barbosa, Almiro José Machado Júnior. Project administration: Denise Fernandes Barbosa, Almiro José Machado Júnior. Resources: Denise Fernandes Barbosa, Almiro José Machado Júnior. Software: Denise Fernandes Barbosa. Supervision: Denise Fernandes Barbosa, Almiro José Machado Júnior. Validation: Denise Fernandes Barbosa, Almiro José Machado Júnior. Visualization: Denise Fernandes Barbosa, Almiro José Machado Júnior. Writing— original draft: Denise Fernandes Barbosa, Almiro José Machado Júnior. Writing—review & editing: Denise Fernandes Barbosa, Almiro José Machado Júnior.
Funding Statement
None
Acknowledgements
We thank the patient for allowing us to use his case in this report. We also thank the Biologix company for providing the required equipment during the COVID-19 pandemic.