The Association between Serotonin Reuptake Inhibitors and Obstructive Sleep Apnea in People with Epilepsy-A Retrospective Analysis
Article information
Trans Abstract
Objectives
Obstructive sleep apnea (OSA) is common in people with epilepsy (PWE), and confers medical and seizure-related consequences when untreated. Positive airway pressure, the gold-standard for OSA management, is limited by tolerability. As serotonin is involved respiratory control and amelioration of seizure-induced respiratory events, this study aims to determine whether serotonin reuptake inhibitors (SRIs) may represent a potential therapeutic option.
Methods
A retrospective study of 100 PWE and OSA ≥18 years of age was conducted. The primary outcome measure was OSA severity as function of SRI use, with rapid eye movement (REM)-related OSA as a secondary outcome.
Results
Older age and depression were more common in those taking an SRI. There was no association between SRIs and OSA severity. However, the SRI group was less likely to have REM-related OSA.
Conclusions
In PWE and OSA, SRI use is associated with reduced risk of REM-related OSA, and may represent a potential management strategy.
Introduction
Obstructive sleep apnea (OSA), characterized by the reduction of airflow during sleep, is a common disease in the general population, with even greater prevalence rates in people with epilepsy (PWE) [1,2]. In addition to the elevated risk of mortality and morbidity associated with untreated OSA, including hypertension, stroke, and metabolic dysfunction, PWE may also exhibit seizure exacerbation and increased epileptogenicity [2]. Though positive airway pressure (PAP) remains the gold-standard for OSA treatment [3], many patients are unable to tolerate this [4], and alternative therapies have been investigated. One potential therapy is the use of serotonin reuptake inhibitors (SRIs).
Serotonin is involved in the control of respiration at multiple sites in the central nervous system (CNS) and peripheral nervous system [5,6], and has demonstrated potential efficacy at reducing OSA severity in animal models and some human studies [7-9]. Increasing evidence also suggests that SRIs may impact breathing-related problems associated with seizures by reducing ictal hypoxemia in medically refractory PWE and seizure-induced respiratory arrest in the Dilute Brown Agouti/1 mouse model of sudden unexpected death in epilepsy [10,11].
As OSA is a prevalent condition associated with significant mortality and morbidity in PWE, investigation of therapies other than continuous PAP (CPAP) is warranted, since a substantial proportion of individuals remain untreated due to intolerance. SRIs, which increase available serotonin levels, may represent such an option. For PWE, the benefits of treatment with a SRI may be even greater, as seizure-related respiratory phenomenon may be ameliorated by SRIs [10,11].
To investigate whether SRIs demonstrate potential as an alternative treatment for OSA in PWE, the objective of this study is to determine whether the use of SRIs is associated with measures of OSA severity.
Methods
A retrospective study of adults ≥18 years of age with epilepsy and OSA was performed at a single academic center. Subjects underwent diagnostic polysomnography (PSG) for suspected sleep apnea between January 1, 2011–January 1, 2016. The study was approved by the site Institutional Review Board.
Potential subjects were identified via electronic search for the International Classification of Diseases (ICD)-10/ICD-9 and Current Procedural Terminology (CPT) codes for sleep apnea, PSG, and epilepsy. Search terms for sleep apnea included the ICD-10 code G47.3, and the ICD-9 codes for OSA (327.23), sleep related nonobstructive alveolar hypoventilation (327.27), obesity hypoventilation syndrome (278.03), sleep related hypoventilation/hypoxemia (327.26), primary central sleep apnea (327.21), Cheyne Stokes breathing pattern (786.04), central sleep apnea/complex sleep apnea (327.27), other sleep apnea (327.29), apnea not elsewhere specified (786.03) and unspecified sleep apnea (327.20). Although only subjects with OSA were included in the study, broader search terms were used to increase the sensitivity to detect patients with OSA, in order to account for any potential ICD misclassification. PSG search terms included ICD-10-Procedure Coding System (PCS) 4A1ZXQZ, ICD-9-Clinical Modification (CM) 89.17 and CPT code 95807 (sleep study, simultaneous recording of ventilation, respiratory effort, electrocardiogram or heart rate, and oxygen saturation, attended by a technologist). Epilepsy codes consisted of ICD-10 G40 and ICD-9-CM 345.
Charts were subsequently reviewed by the principal investigator to confirm that OSA with diagnostic PSG was present during the designated dates. Charts were also individually reviewed to verify the diagnoses of epilepsy (based on the 2014 International League Against Epilepsy operational clinical definition of epilepsy [12]) and OSA (with PSG scoring based on the Center for Medicare and Medicaid Services and OSA defined as per the third edition of the International Classification of Sleep Disorders) [13,14]. Patients who met the above criteria for epilepsy and OSA were included in the final analysis.
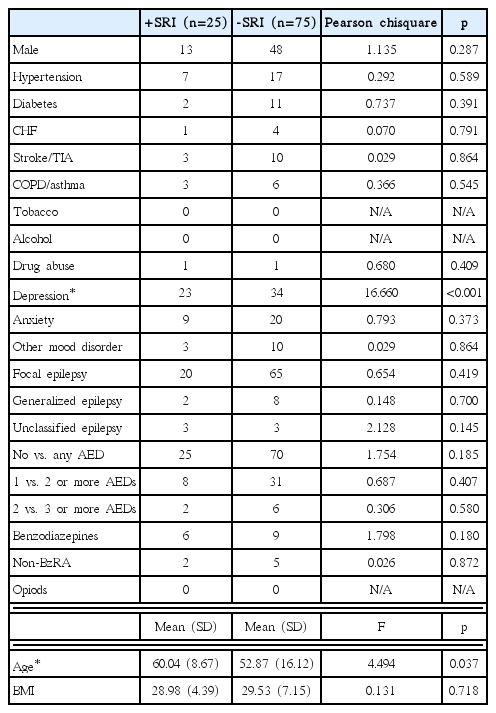
All subjects were divided into two groups by exposure: those taking (+SRI) and those not taking (-SRI) an SRI during PSG. Baseline characteristics were collected as follows: age at the time of diagnostic PSG, gender, body mass index , hypertension, diabetes, congestive heart failure, stroke/transient ischemic attack, chronic obstructive pulmonary disease/asthma, current tobacco use, alcohol abuse, illicit substance abuse, depression, anxiety, other mood disorder, epilepsy syndrome (focal, generalized or unclassified), opioids, benzodiazepines and non-benzodiazepine receptor agonists, and antiepileptic drugs (AEDs).
Based on apnea hypopnea index (AHI), OSA was stratified into four categories of severity: mild (5<AHI<15), mild-tomoderate (5<AHI≤29), moderate (15≤AHI<30) and severe (AHI≥30). Rapid eye movement (REM)-related OSA was defined as REM AHI/non-REM (NREM) AHI≥2 [15]. The primary outcome measure was OSA severity, with REM-related OSA as a secondary outcome.
Statistical analysis
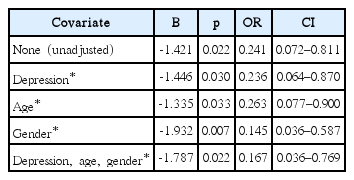
Baseline characteristics were analyzed using Pearson’s χ2 and independent samples t-testing, two-tailed, equal variances not assumed, as appropriate. Binominal logistic regression adjusted for significantly different baseline covariates. A p<0.05 was considered statistically significant.
Results
One hundred and fifty-nine charts were screened. Fifty-nine patients were excluded due to missing polysomnographic data or having a total AHI <5. One hundred eligible patients were therefore included in the final analysis: 25 +SRI and 75 –SRI. The SRIs prescribed included fluoxetine (n=8), escitalopram (n=8), sertraline (n=7), citalopram (n=1), and paroxetine (n=1). The +SRI subjects were older and more likely to have depression; otherwise, there were no other significant differences in baseline characteristics (Table 1).
Data on REM sleep was available in 77/100 subjects: 57/75 (76%) -SRI, and 20/25 (80%) +SRI. REM sleep was not captured during PSG in the remaining 23 subjects. Of the +SRI group, REM-related OSA was present in 4/20 of the +SRI group, and in 29/57 in the -SRI group. The mean time in REM sleep was not significantly different between SRI groups (+SRI: 48.90±28.74 minutes, -SRI: 51.88±32.63 minutes, p=0.718).
There was no association between SRI status and OSA severity, with and without adjustment of significantly different baseline covariates. The mean AHI and standard deviation for the +SRI and -SRI groups was 16.92±3.32 and 24.90± 2.89, respectively, which was not significantly different (p= 0.093, F=2.88, 95% confidence interval -3.27–17.65). However, the +SRI group was less likely to have REM-related OSA compared to the -SRI group. As gender, along with age, has been reported in the literature to interact with REM-related OSA [16], additional adjustment was performed for gender, which was more common in women with REM-related OSA (19/33 vs. 10/44, χ2=9.754, p=0.002). REM-related OSA remained significantly less common in the +SRI group after univariate and multivariate adjustment (Table 2).
Discussion
In this study, REM-related OSA was less likely in PWE who were prescribed an SRI. Though only 77/100 subjects exhibited REM sleep during PSG, the mean duration spent in REM was not different between groups, suggesting that differences in REM time was not a confounder.
Several studies have demonstrated that SRIs ameliorate measures of OSA, although not uniformly [6-9]. However, the impact of SRIs on NREM and REM AHI has been more variable. In crossover trial, 12 patients were randomized to either fluoxetine or protriptyline for 4 weeks each. The baseline AHI decreased from 57±9 to 34±6 with fluoxetine, and from 57±9 to 33±8 with protriptyline, driven by improvement of the NREM AHI [17]. Similarly, in a double-blind, randomized, placebo-controlled crossover study, paroxetine reduced the NREM apnea index by 35%, but no significant effect was found for the hypopnea index or during REM sleep [18]. In contrast, Prasad et al. studied the effect of fluoxetine, ondansetron, or both, versus placebo in patients with OSA, and found a decrease in AHI by approximately 40.5% from baseline for patients on both fluoxetine and ondansetron, which was significant for both NREM and REM sleep [9]. A small trial of mirtazapine significantly reduced NREM and REM AHI in patients with OSA, but no improvement was found when larger trials were conducted [19].
There are several explanations for these mixed findings. Serotonergic effects on respiration are complex, and likely depend upon multiple factors, including anatomical location of response, the degree of activity and selectivity at different receptor subtypes, and duration of exposure [6,20-22]. While the literature generally suggests that SRIs have a positive modulating effect on OSA, the interaction between these features likely accounts for the somewhat inconsistent results that SRIs have demonstrated in human studies of OSA.
Reports on the AHI during REM demonstrate even greater variability. For example, in the studies described above, REM AHI, rather than REM-related OSA, was measured. However, there are many pitfalls in interpreting the significance of REM AHI alone, as it may be influenced by limited duration, which can artificially inflate AHI, or may simply be an epiphenomenon of a high overall AHI in general. For reasons such as these, formal definitions for REM-related OSA have been proposed [23]. In this study, REM-related OSA was defined as per Haba-Rubio et al, in which the overall AHI≥5 and AHI REM/AHI NREM≥2 [15]. This definition minimizes the contribution of NREM AHI. Based on this criteria, 33/77 subjects, or approximately 43%, demonstrated REM-related OSA, which is slightly above the 10–36% prevalence rates reported in the literature [23]. However, as definitions of REM-related OSA vary from study to study, and as prior research has not investigated PWE, previous estimates may not be fully applicable to this cohort.
The significance of REM-related OSA is not clear. Two studies demonstrated that NREM but not REM AHI correlated with excessive daytime sleepiness [24,25], while other smaller series have found no differences in symptomatology, suggesting that REM-related OSA represents a meaningful clinical entity [15,23]. Supporting this, REM-related OSA has been associated with depression symptoms in men [26], hypertension [27], non-dipping of systolic and diastolic blood pressure [28], and impaired long-term glycemic control in patients with type 2 diabetes [29]. In addition, treatment of REM-related OSA may result in positive effects. In a retrospective analysis of 330 adults with REM-related OSA, functional outcome improved post-PAP, as measured by the Epworth Sleepiness Scale, Fatigue Severity Scale, Patient Health Questionnaire-9, and Functional Outcomes Sleep Questionnaire [30].
In PWE, the clinical implications of REM-related OSA are unknown. However, the potential for a significant effect exists. In addition to established associations of untreated OSA, such as hypertension, stroke and metabolic dysfunction [2], several studies have demonstrated that treatment of OSA with CPAP improves seizure frequency and interictal epileptiform activity [31,32]. The mechanism underlying the relationship between SRIs and REM-related OSA in PWE is unclear. However, serotonin is known to control of respiration at multiple sites in the CNS. In the CNS, serotonin stimulates 5-HT2A and/or 5-HT2C upper airway dilator motorneurons, and activates clusters of respiratory neurons in the brainstem mainly via the 5-HT1A, 5-HT2A or 5-HT2C receptors, all of which facilitates upper airway function [5,6]. During sleep, particularly REM sleep, serotonin delivery is reduced, with consequent respiratory depression compared to the waking state. Thus, increased serotonin availability, as may be seen with SRIs, can ameliorate this, and promote normal respiration in those with OSA [6]. In PWE, there is also evidence for serotonergic deficiency in the absence of OSA [33], and thus, there may be an additive effect for PWE with OSA. Greater serotonin availability, such as from SRI use, may similarly ameliorate this effect, but only during stage REM, which represents a vulnerable period for OSA [34,35]. However, this hypothesis is purely speculative, and would require further investigation for elucidation. Future research could include prospective, double-blind, randomized, placebo-controlled trials of SRIs and their effect on OSA in PWE, and could be expanded upon to investigate whether measures of seizure control, such as frequency, severity or number of antiseizure medications, are associated with REM-related OSA.
There are several limitations to this research. Although diagnostic accuracy was controlled for by confirmation of OSA and epilepsy diagnoses via individual chart review, and significantly different baseline co-morbidities were adjusted for, the limitations of retrospective research remain. ICD 10/9 and CPT codes for diagnoses other than OSA and epilepsy were not verified, and may have been inaccurate. The indication for SRI prescriptions was not always clear. Adjustment for multiple potential confounders was not performed due to small sample size and incomplete data. This included seizure frequency, type(s) of AEDs used, SRI duration of use, SRI dose, type of SRI used, medication adherence, and use of other neuropsychiatric drugs and drug classes. Only associations could be established. As a single site study with a limited number of subjects, the results may not be generalizable, and sampling error may have skewed results.
In conclusion, in PWE and OSA, SRI use is associated with reduced risk of REM-related OSA, and may represent a potential management strategy.