Periodic Limb Movements During Sleep Associated with Human T-Lymphotropic Virus Type I-Associated Myelopathy
Article information
Abstract
Periodic limb movements during sleep (PLMS) are frequently observed in the general population, although such movements may be associated with a variety of medical and neurological disorders. Human T-lymphotropic virus type I-associated myelopathy (HAM) is a rare progressive disease in which abnormalities are rarely observed on spinal images. We present the case of a 55-year-old woman with PLMS who was later diagnosed with HAM. The current case indicates that HAM can be considered a possible cause of PLMS.
Periodic limb movements during sleep (PLMS) is characterized by involuntary, stereotyped, and repetitive limb movements that commonly occur during sleep [1,2]. PLMS affects 4–10% of the general adult population and may be associated with a variety of conditions, such as uremia, iron deficiency, peripheral neuropathy, radiculopathy, or spinal cord lesions [1]. Patients with human T-lymphotropic virus type I (HTLV-I) -associated myelopathy (HAM) exhibit slowly progressive spastic paraparesis, impairment of gait, autonomic dysfunction (e.g., bladder symptoms), and reduced quality of life [3]. PLMS is extremely rare in patients with HAM: only one case of PLMS has been previously reported in a Japanese carrier of HTLV-I [4]. In the present report, we discuss the case of a patient diagnosed with HAM who initially presented with PLMS.
Case Report
A 55-year-old woman visited our hospital for evaluation of involuntary nocturnal movements of the left leg, which had developed 5 months prior to her initial visit. She reported having difficulty initiating or maintaining sleep due to the symptom, though she did not experience symptoms of restless legs syndrome. At the time of her initial visit, she had been taking anti-rheumatic drugs such as tacrolimus, hydroxychloroquine, and methotrexate for 10 years. She reported no recent history of travel or febrile illness prior to the onset of symptoms or relevant family history. No significant abnormalities were noted upon neurological examination, with the exception of mild postural tremor in both hands.
The patient underwent overnight video polysomnography (VPSG, COMET PSG; Grass Technologies, Twin 4.5.2 Software, Warwick, RI, USA) 6 months following the onset of symptoms. The VPSG revealed the presence of regular, repetitive, and dorsiflexive movements in the left big toe and ankle (Fig. 1), consistent with a diagnosis of PLMS based on the criteria outlined by the American Academy of Sleep Medicine [5]. VPSG was performed from 11:10 PM until 5:40 AM the next day. Total sleep time, sleep onset latency, and sleep efficiency were 248.5 min, 80.5 min, and 63.9%, respectively. The respiratory distress index was 4.1/h. Total PLMS and PLMS-index were 434 and 101.9/h, respectively.
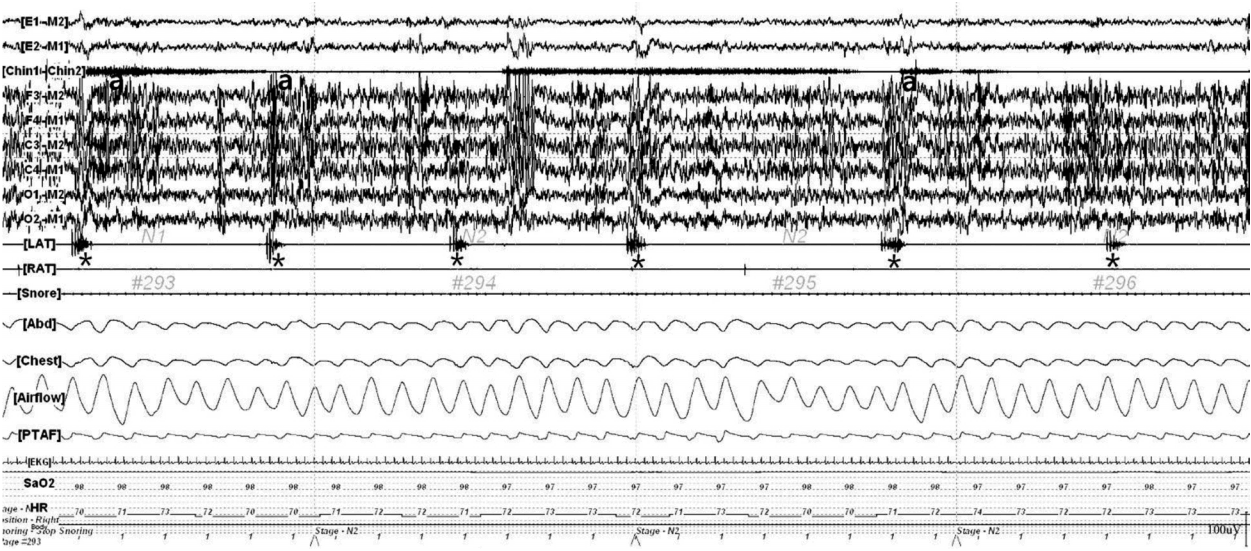
This 120-s epoch polysomnogram demonstrates six periodic movements of the left leg (*). The movements lasted for 1.8 to 2 s and occurred at intervals of 16 to 24 s. These movements are often associated with electroencephalographic arousal (a). Top 2 channels: electrooculogram, Chin 1-Chin 2: chin electromyogram, six channels consisting of F3-M2, F4-M1, C3-M2, C4-M1, O1-M2, O2-M1: electroencephalogram, LAT: electromyogram on left anterior tibialis muscle, RAT: electromyogram on right anterior tibialis muscle, Snore: snore sensor, Abd: abdomen respiratory effort channel, Chest: chest respiratory effort channel, Airflow: oro-nasal thermal sensor; PTAF: pressure transducer air-flow, EKG: electrocardiography, SaO2: oxyhemoglobin saturation by pulse oximetry, HR: heart rate.
One month following her initial visit to our hospital, no change was observed in the patient’s PLMS symptoms, though she reported experiencing gait disturbance and feelings of bladder fullness. At that time, neurological examination revealed decreased motor power [Medical Research Council (MRC) grade IV] with spasticity in both legs. She had brisk deep tendon reflexes in both knees with bilateral positive Babinski signs; however, no sensory deficits were observed. Magnetic resonance images (MRIs) of the brain and spinal cord were normal in appearance.
Examination via nerve conduction tests and electromyography revealed no abnormal findings. Somatosensory evoked potentials (SEPs) after median nerve stimulation were also normal. However, SEPs after stimulation of the bilateral posterior tibial nerves exhibited prolonged latencies of P1, LN1-P1, and TN1-P1: P1, LN1, and TN1 wave-forms arose from the subcortex, third lumbar, and 12th thoracic regions, respectively.
Routine blood laboratory values were also normal. Extensive blood tests for various causes of myelopathy exhibited normal results for thyroid function, the presence of human immunodeficiency virus antibodies, rapid plasma regain, vitamin B12 levels, levels of very-long-chain fatty acids, and arylsulfatase A levels. Genetic tests for spastic paraplegia gene (SPG) 3A, SPG4, and X-linked adrenoleukodystrophy gene (ABCD1) were also normal. Cerebrospinal fluid (CSF) analysis revealed mild lymphocytic pleocytosis (8/μL) as well as mildly elevated levels of protein (146 mg/dL).
Chemiluminescent magnetic immunoassay (CMIA) indicated that the patient was positive for serum antibody to HTLV-I (×156.01), leading to a diagnosis of HAM. The patient was treated with intravenous methylprednisolone for 5 days, followed by treatment with oral prednisolone. Following steroid treatment, the patient’s symptoms gradually improved. Two months after initiating steroid treatment, she reported no symptoms of involuntary nocturnal leg movements or bladder fullness. Neurological examinations revealed an improvement in the motor power of both legs (MRC IV+). Though she reported continued experiences of gait disturbance, considerable improvement was observed following steroid treatment.
Discussion
Our patient initially presented with PLMS prior to the clinical manifestation of HAM symptoms. No other cause of myelopathy was identified following extensive examination. Although MRI revealed no definitive spinal cord lesions, the clinical manifestation and results of the electrophysiological analysis were consistent with a diagnosis of myelopathy.
HAM is a type of chronic progressive meningomyelitis affecting both the white and gray matter, resulting in axonal damage in the spinal cord [6]. Some reports have indicated that patients with HTLV-I-associated conditions exhibit spinal cord atrophy on MRIs [3]. Although patients with rapidly progressive diseases are more likely to show high signal intensity and contrast enhancement lesions on MR images of the spinal cord [3], most patients exhibit no abnormalities on spinal images [3]. Virus-specific immune responses triggered by HTLV-I-infected T cells are considered to play important roles in the pathomechanisms of HAM [6]. Therefore, immunosuppressive agents are used to treat patients with HAM.
The anatomic location of the lesion remains unclear in PLMS, though various studies indicate that both central and peripheral lesions in the nervous system may be associated with PLMS [3]. PLMS is usually implicated in spinal cord lesions associated with compression, injury, vascular disease, and demyelination [7]. PLMS phenotypically presents as dorsiflexion of the ankle and big toe, resembling the Babinski reflex observed in patients with pyramidal tract lesions [4]. In a previous experimental study, researchers revealed that PLMS was very similar to the flexor withdrawal reflex [8]. Patients with PLMS exhibited increased excitability in flexor withdrawal reflexes following electrical stimulation of the medial plantar nerve during sleep [9]. PLMS may be closely involved in the spinal circuitry of flexor withdrawal reflexes, which are regulated by the dorsal reticulospinal tract (DRT) [4]. PLMS is presumably attributed to interruption of the portion of the DRT located just dorsolateral to the corticospinal tract [10]. Histopathological studies of HAM have demonstrated perivascular and parenchymal lymphocytic infiltration in the lateral column of the spinal cord [6]. Such findings therefore indicate that HAM is a possible cause of PLMS.
In order to confirm the diagnosis of HAM, additional tests such as serum Western blot or polymerase chain reaction (PCR) analysis are required in patients with positive screening tests (e.g., CMIA, enzyme immunoassay) [6]. The presence of HTLV-I antibodies must be confirmed in both the CSF and blood [6]. Unfortunately, we did not perform CSF antibody, blood Western blotting, or PCR in the present case.
In the present report, we illustrated the case of a patient with PLMS associated with HAM. This patient initially presented with PLMS, prior to the clinical manifestation of HAM. As only one previous case of PLMS has been reported in a Japanese carrier of HTLV-I, this manner of presentation is exceedingly rare. Testing for HAM should be considered in patients with PLMS, particularly those with myelopathy symptoms whose spinal cord images reveal no abnormalities.