Chee, MBBS, and FRCP (Edin): Imaging the Sleep Deprived Brain: A Brief Review
Introduction
Functional magnetic resonance imaging (fMRI) is a highly versatile tool used to study neurobehavioral alterations associated with sleep deprivation (SD). Task-related fMRI is the most widely used technique and an impressive list of cognitive domains has been evaluated using this technique ( Table 1). 1-40 fMRI measures relative change in blood oxygenation level dependant (BOLD) signal in capillaries and venules adjacent to neuronal clusters whose firing rate and consequently, synaptic potentials are modulated by task performance. An increase in MR signal occurs as a result of a relatively disproportionate elevation in blood flow relative to oxygen consumption in response to sensory stimulation and/or task performance. In addition to task-related activation, the evaluation of task-related deactivation where signal changes fall below baseline levels can be evaluated. 1,18,25,30,41
Blood oxygenation level dependant imaging measures relative changes in blood flow, but does not ascertain absolute blood flow. Quantification of blood flow may occasionally be useful, for example, to study time-on-task effects, 42 and other phenomena whose observation requires signal stability over several minutes as opposed to several seconds. Such measurements can be obtained using a variety of arterial spin labeling (ASL) techniques that have different levels of precision. 43,44 A disadvantage of ASL is its inferior signal to noise ratio relative to BOLD imaging. Additionally, the requirement for block sampling also makes it impossible to perform event-related designs that are important in separating out trials where the subject may have been asleep. 8
The evaluation of functional connectivity, conducted by assessing signal covariation in pairs of regions, or by determining the extent to which signal in a ‘target’ region interacts with that of a ‘seed’ region according to state/task context provides additional characterization of altered physiology. The latter method, known as psychophysiological Interaction-PPI has been applied in studies evaluating selective attention, 12,45,46 the processing of emotional pictures as well as executive function/working memory. 29,35
In addition to fMRI studies designed to evaluate signal changes in response to task performance, it may be informative to evaluate ‘resting-state’ activity or intrinsic functional connectivity. 47,48 This refers to the identification of regions showing synchronous low frequency oscillations (0.1-0.01 Hz) in BOLD signal that are not time locked to task performance or sensory stimulation. Studies of this type in sleeping individuals have shown changes in connectivity within the default mode network (DMN) alluded to earlier. 49,50 The first study evaluating resting state networks in the setting of SD found selective reductions in DMN functional connectivity and reduced anti-correlation with low frequency oscillations in the ‘task-positive’ network ( Fig. 1). 18 Analyses of resting state data hold promise of being informative of alterations in brain function without requiring motivated performance on the part of a participant. 51 Related to functional connectivity, MRI in the form of diffusion tensor imaging (DTI) can be used to evaluate white matter connectivity. Strangely, only one study to date has used DTI to study sleep deprived individuals. 52
Combining electroencephalography (EEG) and fMRI in SD or sleep related studies is primarily motivated by the need to monitor sleep stage although the high temporal resolution of EEG is also well suited to study transient phenomena like lapsing. 53 The neural correlates of spindles and slow waves have been studied using this technique. 54-56 In addition, fMRI guided repetitive transcranial magnetic stimulation has been used to evaluate the therapeutic potential of transcranial magnetic stimulation in alleviating SD. 28
Effects of Task Difficulty, Cognitive Domain
Tested and Inter-Individual Differences
The results of behavioral studies suggested that cognitive domain engaged and task difficulty might affect neural response to SD. 21 This motivated subsequent studies probing working memory to examine the effect of manipulating task difficulty and/or item load. 1,2,57 Although the specific areas most affected by SD differ somewhat across these studies, a common set of findings concerns the decrease in higher visual cortex and fronto-parietal activation when performance declined during SD. In contrast, activation is relatively preserved when performance is maintained. The notion that increased task difficulty might elicit ‘compensatory’ frontal activation was then independently demonstrated in a study evaluating logical reasoning. 23
As experience in fMRI cumulated with studies that recruited at least 20 subjects, it became evident that inter-individual differences in response to SD could be identified reliably using imaging. A few studies suggested that a single imaging study conducted in the rested state might predict vulnerability to performance decline in the tested domain whereas later studies found that a shift in activation across state may be more reliable as a marker of inter-individual differences in performance when sleep deprived. 4,9,11,24,26
A wealth of behavioral data collected on behavior in sleep deprived persons suggests the loss of vigilance or sustained attention to be the most prominent deficit encountered in SD. 14 The failure of attention and the possible maladaptive consequences of endogenous efforts to sustain wakefulness in the face of mounting sleep pressure has been a central idea. A cogent illustration of the vital role played by attention in supporting cognitive performance comes from an experiment originally designed to evaluate visual working memory capacity in SD. 5 Participants in this study were briefly shown an array of colored squares and seconds later had to verify if the test item was of a color shown in the array. The number of differently colored squares shown varied from 1 to 8 squares. The key finding was that during SD, even when memory capacity and perceptual load were not taxed, task-related activation in brain regions mediating top-down control of attention and visual perception in extrastriate visual cortex was significantly diminished ( Fig. 2). This indicated that a more global curtailment of processing resources rather than storage limitation was problematic. As both working memory and attention are intertwined processes and engage similar cortical and subcortical areas, 58,59 the parametric variation of visual memory and visual item load across multiple levels in two parallel sets of scans were instrumental in proving the point. Other studies probing the effects of SD on selective attention used visual tracking or visual picture selection paradigms. 12,37,46 They arrived at convergent observations regarding the attenuation of top-down control of attention together with diminished engagement of the visual extrastriate cortex. Together with deficits in responding to stimuli, attenuated activation of fronto-parietal and visual extrastriate cortex also occurs in the preparatory period prior to stimulus appearance. 15 In comparison to its effects on cortical activation the effects of SD on subcortical activation are more complex. For example, the thalamus, which plays an important role in mediating arousal and attention, shows decreased glucose metabolism during SD if measurement is averaged over several minutes. 60 However, for individual events, activation can either be higher or lower than during rested wakefulness depending on whether the sleep deprived volunteer is performing adequately or lapsing. 8,37
Evaluating Countermeasures
A majority of persons show decline in cognitive performance after 24 h of SD. fMRI has been used to track the efficacy of countermeasures against, for example, to evaluate the effect of modafinil, 39 and donepezil. 9,10 In these studies, the effect of drug in the well-rested state was negligible but was evident in the sleep-deprived state in some volunteers. In the donepezil trials, improvement was positively correlated with the magnitude of performance decline when undergoing SD on placebo in both short-term memory and episodic memory experiments in the same subjects. The smaller trial involving modafanil evidenced increases in both cortical and subcortical activation in volunteers sleep deprived on modafanil. Repetitive transcranial magnetic stimulation has been reported to improve verbal working memory in sleep deprived volunteers when applied to the right ‘upper middle occipital’ region that was part of the network associated with SD induced performance impairment. 28 Improvement correlated with the extent to which performance declined during SD in sham stimulations. In contrast no benefit was observed when stimulating the midline parietal region, part of a network of areas showing reduced task-related activation in SD, or when the lower left middle occipital gyrus which was not part this network.
Affective Aspects of Behavior-Emotional Processing and Decision-Making
Most of the tasks used in experimental studies engage a fr-onto-parietal network involved in the control of attention and do not evaluate affective changes that constitute an important aspect of behavioral alteration in SD. Brain structures involved in affective processing include the amygdala, striatum, ventromedial prefrontal cortex and the insula. These structures can be expected to show altered activation if a state-related change in affect is present.
In one study, where participants graded the emotionality of faces, SD enhanced amygdala responses to negative pictures. 29 Accompanying this change was reduced functional connectivity between the medial prefrontal cortex and amygdala ( Fig. 3). In a second study, 13 volunteers remembered neutral faces over a brief interval during which distracter pictures were presented. The distractors could be neutral, negative emotional or noise patterns. Amygdala activation during the maintenance period was higher for emotional distracters than neutral distracters in both states although maintenance activity was overall, lower in the SD state for both conditions ( Fig. 3). Face memoranda were less well maintained in working memory to the extent that SD reduced functional connectivity between ventromedial and dorsolateral frontal areas and the amygdala. 13 Responses to emotional stimuli during SD can thus be perturbed by disrupting the transmission of top-down control signals from regions involved in valuation and/or cognitive control. In one experiment, negative stimuli were better recognized after SD than neutral or positive stimuli. The recollection of negative and positive stimuli was correlated with greater functional connectivity between medial frontal regions and the hippocampus when subjects had a normal night of sleep compared to when they were sleep-deprived. 61
In another experiment, the bias towards processing emotional stimuli over neutral ones was extended to positive stimuli. SD also caused volunteers to increase the number of neutral pictures that were rated positively. 32 This finding was related to enhanced hippocampal to medial frontal and hippocampal to orbitofrontal connectivity in the SD state.
Opportunities for Research
A critical gap in our current knowledge of the mechanisms underpinning altered behavior in SD lies in the piecemeal use of functional imaging in this state whereby a single cognitive domain is tested at a time. Behavioral studies show that SD may affect performance unevenly across different cognitive domains. 62,63 It remains a open challenge to execute withinsubject studies involving multiple, sensitive, short-duration, tasks that probe dissociable cognitive domains. 64
While total SD is convenient to study in a laboratory setting, most persons encounter the effects of sleep loss through chronic sleep restriction and sleep fragmentation. 65 Partial SD studies where sleep is restricted to between 3-6 hours of time in bed per night suggest that the relationship between hours of sleep and cognition is not linear. 66,67 The rate of build up of slow wave activity during sustained wakefulness and its dissipation following recovery sleep also does not correspond well with behavioral performance alteration suggesting that different measures provide independent information regarding changes occurring in SD. It remains of interest to study how functional imaging changes evolve during partial SD-such studies can provide new understanding of compensatory brain network dynamics during the process of chronic partial sleep restriction. 66
Imaging genomics seeks to identify genes that influence brain, cognition and risk for disease. For example, the candidate gene approach was applied to study the effect of Per 3 5/5 on n-back task performance in subjects undergoing total SD. Widespread relative reductions in task related activation were found in frontal, parietal and occipital regions in this vulner-able group when they were sleep deprived. 35 The intriguing behavioral results were not replicated when a different set of sleep restricted (not total SD) subjects were studied leaving room for future studies to clarify. 68
Sleep deprivation provides a unique opportunity to perturb brain networks of healthy individuals in a manner that degrades performance in a reversible manner. Several similarities between the behavioral deficits in SD and cognitive aging have been highlighted. 69,70 There are also functional imaging parallels that remain to be exploited in future studies. 1,71 The attraction of this approach is that it may allow conspecific evaluation of cognitive enhancers in a relatively lower risk setting, keeping in mind that the target group of older adults may be less resilient to adverse effects should these occur.
Acknowledgments
This work was supported by grants awarded to M.C. from the Defence Science and Technology Agency Singapore (POD0713897) and the National Research Foundation Singapore (STaR Award). Substantial portions of this review were derived from Ch. 16 of ‘Neuroimaging of Sleep and Sleep Disorders’ edited by Nofzinger, Maquet and Thorpy; Cambridge University Press 2013.
Figure 1.
Main effect of state on default mode network (DMN) functional connectivity. Schematic showing a significant main effect of state on DMN functional connectivity between 3 node pairs using a seed based analysis; left inferior parietal lobe (LIPL) and dorsal medial prefrontal cortex (dMPFC), LIPL and ventromedial prefrontal cortex (vMPFC), and LIPL and posterior cingulate cortex (PCC; Bonferroni corrected p<0.05/21=0.002). Adapted from De Havas JA, Parimal S, Soon CS, Chee MW. Neuroimage 2012;59:1745- 1751.18 LLTC: left lateral temporal cortex, RLTC: right lateral temporal cortex, RIPL: right inferior parietal lobe. 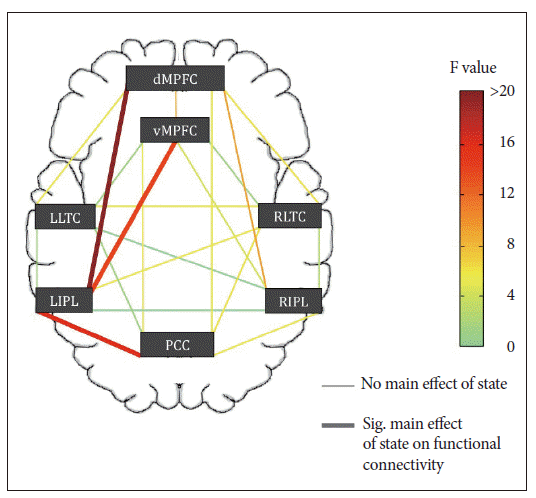
Figure 2.
Deficits in attention underlie functional imaging changes when visual short-term memory is tested in the sleep-deprived state. Taskrelated activation of the intraparietal sulcus increases with memory load--but not visual item load when memory is not engaged--in both rested wakefulness (RW) and sleep deprivation (SD). In the sleep-deprived state, even when visual short-term memory is not taxed (e.g., low test item load or no load on memory), decrements in activation are observed. This implicates a fundamental deficit in attention rather than one of storage capacity or memory. Adapted from Chee MW, Chuah YM. Proc Natl Acad Sci U S A 2007;104:9487-9492.5
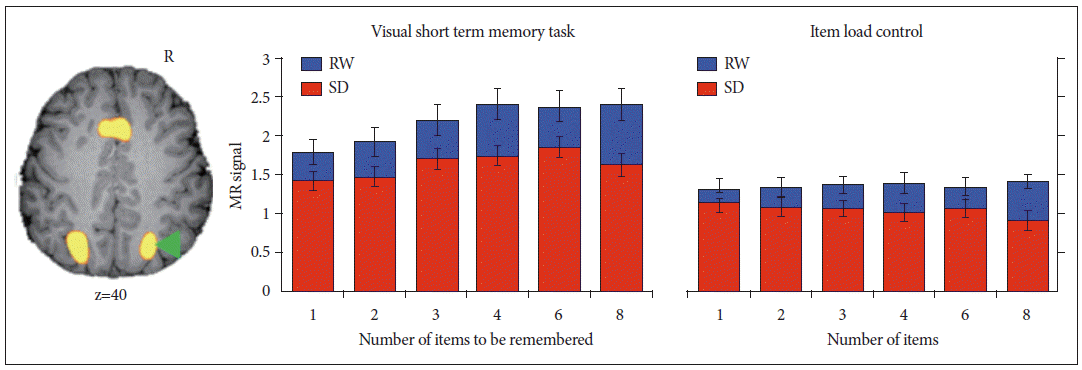
Figure 3.
Right amygdala activity was elevated in response to emotional distracters relative to neutral distracters at rested wakefulness (RW). A state (rested wakefulness, sleep deprivation) by Condition (Neutral, Emotional) repeated-measures ANOVA conducted on averaged activation within this region of interest indicated marginal decreases in amygdala activation following sleep deprivation. Critically, state-related change in amygdala activation correlated with the corresponding alteration of emotional distractibility while no parallel effect was present for neutral distracters. Adapted from Chuah LY, Dolcos F, Chen AK, Zheng H, Parimal S, Chee MW. Sleep 2010;33:1305-1313.13 BOLD: blood oxygenation level dependant. 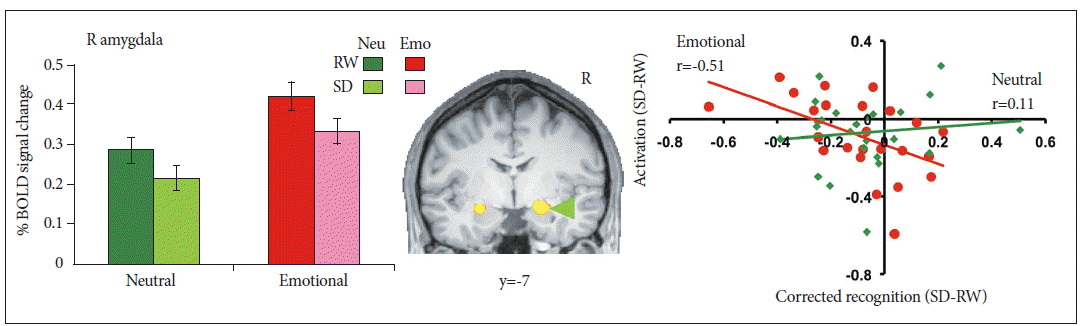
Table 1.
fMRI studies involving sleep deprivation
|
Cognitive domain |
Reference |
|
Working memory |
Bell-McGinty et al.22
|
|
Habeck et al.57
|
|
Chee and Choo1
|
|
Choo et al.2
|
|
Caldwell et al.24
|
|
Mu et al.26
|
|
Mu et al.27
|
|
Chee et al.4
|
|
Thomas and Kwong39
|
|
Lim et al.6
|
|
Luber et al.28
|
|
Vandewalle et al.35
|
|
Attention (sustained or divided) |
Portas et al.40
|
|
Thomas et al.60
|
|
Drummond et al.25
|
|
Chee et al.8
|
|
Tucker et al.31
|
|
Attention (selective) |
Mander et al.33
|
|
Tomasi et al.37
|
|
Volkow et al.38
|
|
Chee et al.12
|
|
Lim et al.46
|
|
Chee and Tan11
|
|
Chee et al.15
|
|
Kong et al.16
|
|
Visual short-term memory |
Chee and Chuah5
|
|
Chuah and Chee9
|
|
Logical reasoning |
Drummond et al.23
|
|
Inhibition (Go/No-Go) |
Chuah et al.3
|
|
Risky decision making |
Venkatraman et al.7
|
|
Venkatraman et al.17
|
|
Emotional Processing |
Yoo et al.29
|
|
Sterpenich et al.61
|
|
Chuah et al.13
|
|
Gujar et al.32
|
|
Verbal learning/Verbal episodic memory |
Drummond et al.20
|
|
Yoo et al.34
|
|
Chuah et al.10
|
|
Default mode/Resting state |
Gujar et al.30
|
|
De Havas et al.18
|
REFERENCES
1. Chee MW, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci 2004;24:4560-4567.   2. Choo WC, Lee WW, Venkatraman V, Sheu FS, Chee MW. Dissociation of cortical regions modulated by both working memory load and sleep deprivation and by sleep deprivation alone. Neuroimage 2005;25:579-587.   3. Chuah YM, Venkatraman V, Dinges DF, Chee MW. The neural basis of interindividual variability in inhibitory efficiency after sleep deprivation. J Neurosci 2006;26:7156-7162.   4. Chee MW, Chuah LY, Venkatraman V, Chan WY, Philip P, Dinges DF. Functional imaging of working memory following normal sleep and after 24 and 35 h of sleep deprivation: correlations of fronto-parietal activation with performance. Neuroimage 2006;31:419-428.   5. Chee MW, Chuah YM. Functional neuroimaging and behavioral correlates of capacity decline in visual short-term memory after sleep deprivation. Proc Natl Acad Sci U S A 2007;104:9487-9492.    6. Lim J, Choo WC, Chee MW. Reproducibility of changes in behaviour and fMRI activation associated with sleep deprivation in a working memory task. Sleep 2007;30:61-70.  7. Venkatraman V, Chuah YM, Huettel SA, Chee MW. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep 2007;30:603-609.  8. Chee MW, Tan JC, Zheng H, et al. Lapsing during sleep deprivation is associated with distributed changes in brain activation. J Neurosci 2008;28:5519-552.   9. Chuah LY, Chee MW. Cholinergic augmentation modulates visual task performance in sleep-deprived young adults. J Neurosci 2008;28:11369-11377.   10. Chuah LY, Chong DL, Chen AK, et al. Donepezil improves episodic memory in young individuals vulnerable to the effects of sleep deprivation. Sleep 2009;32:999-1010.   11. Chee MW, Tan JC. Lapsing when sleep deprived: neural activation characteristics of resistant and vulnerable individuals. Neuroimage 2010;51:835-843.   12. Chee MW, Tan JC, Parimal S, Zagorodnov V. Sleep deprivation and its effects on object-selective attention. Neuroimage 2010;49:1903-1910.   13. Chuah LY, Dolcos F, Chen AK, Zheng H, Parimal S, Chee MW. Sleep deprivation and interference by emotional distracters. Sleep 2010;33:1305-1313.    14. Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull 2010;136:375-389.    15. Chee MW, Goh CS, Namburi P, Parimal S, Seidl KN, Kastner S. Effects of sleep deprivation on cortical activation during directed attention in the absence and presence of visual stimuli. Neuroimage 2011;58:595-604.    16. Kong D, Soon CS, Chee MW. Reduced visual processing capacity in sleep deprived persons. Neuroimage 2011;55:629-634.   17. Venkatraman V, Huettel SA, Chuah LY, Payne JW, Chee MW. Sleep deprivation biases the neural mechanisms underlying economic preferences. J Neurosci 2011;31:3712-3718.   18. De Havas, Parimal S, Soon CS, Chee MW. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. Neuroimage 2012;59:1745-1751.   19. Drummond SP, Brown GG, Stricker JL, Buxton RB, Wong EC, Gillin JC. Sleep deprivation-induced reduction in cortical functional response to serial subtraction. Neuroreport 1999;10:3745-3748.   20. Drummond SP, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB. Altered brain response to verbal learning following sleep deprivation. Nature 2000;403:655-657.   21. Drummond SP, Brown GG. The effects of total sleep deprivation on cerebral responses to cognitive performance. Neuropsychopharmacology 2001;25(5 Suppl):S68-S73.   22. Bell-McGinty S, Habeck C, Hilton HJ, et al. Identification and differential vulnerability of a neural network in sleep deprivation. Cereb Cortex 2004;14:496-502.   23. Drummond SP, Brown GG, Salamat JS, Gillin JC. Increasing task difficulty facilitates the cerebral compensatory response to total sleep deprivation. Sleep 2004;27:445-451.  24. Caldwell JA, Mu Q, Smith JK, et al. Are individual differences in fatigue vulnerability related to baseline differences in cortical activation? Behav Neurosci 2005;119:694-707.   25. Drummond SP, Bischoff-Grethe A, Dinges DF, Ayalon L, Mednick SC, Meloy MJ. The neural basis of the psychomotor vigilance task. Sleep 2005;28:1059-1068.  26. Mu Q, Mishory A, Johnson KA, et al. Decreased brain activation during a working memory task at rested baseline is associated with vulnerability to sleep deprivation. Sleep 2005;28:433-446.  27. Mu Q, Nahas Z, Johnson KA, et al. Decreased cortical response to verbal working memory following sleep deprivation. Sleep 2005;28:55-67.  28. Luber B, Stanford AD, Bulow P, et al. Remediation of sleep-deprivation- induced working memory impairment with fMRI-guided transcranial magnetic stimulation. Cereb Cortex 2008;18:2077-2085.    29. Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep--a prefrontal amygdala disconnect. Curr Biol 2007;17:R877-R878.   30. Gujar N, Yoo SS, Hu P, Walker MP. The unrested resting brain: sleep deprivation alters activity within the default-mode network. J Cogn Neurosci 2010;22:1637-1648.    31. Tucker AM, Rakitin BC, Basner RC, Gazes Y, Steffener J, Stern Y. fMRI activation during failures to respond key to understanding performance changes with sleep deprivation. Behav Brain Res 2011;218:73-79.    32. Gujar N, Yoo SS, Hu P, Walker MP. Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. J Neurosci 2011;31:4466-4474.    33. Mander BA, Santhanam S, Saletin JM, Walker MP. Wake deterioration and sleep restoration of human learning. Curr Biol 2011;21:R183-R184.    34. Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nat Neurosci 2007;10:385-392.   35. Vandewalle G, Archer SN, Wuillaume C, et al. Functional magnetic resonance imaging-assessed brain responses during an executive task depend on interaction of sleep homeostasis, circadian phase, and PER3 genotype. J Neurosci 2009;29:7948-7956.   36. Mander BA, Reid KJ, Davuluri VK, et al. Sleep deprivation alters functioning within the neural network underlying the covert orienting of attention. Brain Res 2008;1217:148-156.    37. Tomasi D, Wang RL, Telang F, et al. Impairment of attentional networks after 1 night of sleep deprivation. Cereb Cortex 2009;19:233-240.    38. Volkow ND, Tomasi D, Wang GJ, et al. Hyperstimulation of striatal D2 receptors with sleep deprivation: implications for cognitive impairment. Neuroimage 2009;45:1232-1240.    39. Thomas RJ, Kwong K. Modafinil activates cortical and subcortical sites in the sleep-deprived state. Sleep 2006;29:1471-1481.  40. Portas CM, Rees G, Howseman AM, Josephs O, Turner R, Frith CD. A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J Neurosci 1998;18:8979-8989.  41. McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci 2003;15:394-408.   42. Lim J, Wu WC, Wang J, Detre JA, Dinges DF, Rao H. Imaging brain fatigue from sustained mental workload: an ASL perfusion study of the time-on-task effect. Neuroimage 2010;49:3426-3435.    43. Buxton RB. Quantifying CBF with arterial spin labeling. J Magn Reson Imaging 2005;22:723-726.   44. Aguirre GK, Detre JA, Zarahn E, Alsop DC. Experimental design and the relative sensitivity of BOLD and perfusion fMRI. Neuroimage 2002;15:488-500.   45. Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 1997;6:218-229.   46. Lim J, Tan JC, Parimal S, Dinges DF, Chee MW. Sleep deprivation impairs object-selective attention: a view from the ventral visual cortex. PLoS One 2010;5:e9087.    47. Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995;34:537-541.   48. Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 2005;360:1001-1013.    49. Horovitz SG, Braun AR, Carr WS, et al. Decoupling of the brain’s default mode network during deep sleep. Proc Natl Acad Sci U S A 2009;106:11376-11381.    50. Sämann PG, Tully C, Spoormaker VI, et al. Increased sleep pressure reduces resting state functional connectivity. MAGMA 2010;23:375-389.   51. Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci 2010;4:8.    52. Rocklage M, Williams V, Pacheco J, Schnyer DM. White matter differences predict cognitive vulnerability to sleep deprivation. Sleep 2009;32:1100-1103.   53. Rasch B, Büchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science 2007;315:1426-1429.   54. Dang-Vu TT, Desseilles M, Laureys S, et al. Cerebral correlates of delta waves during non-REM sleep revisited. Neuroimage 2005;28:14-21.   55. Dang-Vu TT, Schabus M, Desseilles M, et al. Spontaneous neural activity during human slow wave sleep. Proc Natl Acad Sci U S A 2008;105:15160-15165.    56. Schabus M, Dang-Vu TT, Albouy G, et al. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc Natl Acad Sci U S A 2007;104:13164-13169.    57. Habeck C, Rakitin BC, Moeller J, et al. An event-related fMRI study of the neurobehavioral impact of sleep deprivation on performance of a delayed-match-to-sample task. Brain Res Cogn Brain Res 2004;18:306-321.   58. Awh E, Vogel EK, Oh SH. Interactions between attention and working memory. Neuroscience 2006;139:201-208.   59. Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 2002;3:201-215.   60. Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive
performance impairments during sleepiness. I. Effects of 24 h of
sleep deprivation on waking human regional brain activity. J Sleep Res 2000;9:335-352.   61. Sterpenich V, Albouy G, Boly M, et al. Sleep-related hippocampo-cortical interplay during emotional memory recollection. PLoS Biol 2007;5:e282.    62. Leproult R, Colecchia EF, Berardi AM, Stickgold R, Kosslyn SM, Van Cauter. Individual differences in subjective and objective alertness during sleep deprivation are stable and unrelated. Am J Physiol Regul Integr Comp Physiol 2003;284:R280-R290.   63. Van Dongen, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss:evidence of trait-like differential vulnerability. Sleep 2004;27:423-433.  64. Drobyshevsky A, Baumann SB, Schneider W. A rapid fMRI task battery for mapping of visual, motor, cognitive, and emotional function. Neuroimage 2006;31:732-744.    65. Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol 2009;29:320-339.    66. Van Dongen, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 2003;26:117-126.  67. Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res 2003;12:1-12.   68. Goel N, Banks S, Mignot E, Dinges DF. PER3 polymorphism predicts cumulative sleep homeostatic but not neurobehavioral changes to chronic partial sleep deprivation. PLoS One 2009;4:e5874.    69. Harrison Y, Horne JA, Rothwell A. Prefrontal neuropsychological effects of sleep deprivation in young adults--a model for healthy aging? Sleep 2000;23:1067-1073.  70. Tucker AM, Stern Y, Basner RC, Rakitin BC. The prefrontal model revisited: double dissociations between young sleep deprived and elderly subjects on cognitive components of performance. Sleep 2011;34:1039-1050.    71. Kong D, Soon CS, Chee MW. Functional imaging correlates of impaired distractor suppression following sleep deprivation. Neuroimage 2012;61:50-55.  
|
|













