INTRODUCTION
Obstructive sleep apnea (OSA), a well-recognized risk factor for cardiovascular morbidity and mortality, is an independent predictor of atrial fibrillation (AF) [1]. OSA and AF share multiple risk factors, such as age, sex, obesity, hypertension, and heart failure. Recent studies have suggested that OSA promotes AF through structural and electrical atrial remodeling [2]. A recent meta-analysis showed that continuous positive airway pressure (CPAP) therapy reduces AF recurrence risk by 42% in patients with OSA [3]. However, whether CPAP can prevent AF or convert AF to normal sinus rhythm remains controversial. In this case report, we describe a patient with severe OSA and AF, whose AF was converted to normal sinus rhythm after the application of CPAP therapy.
CASE REPORT
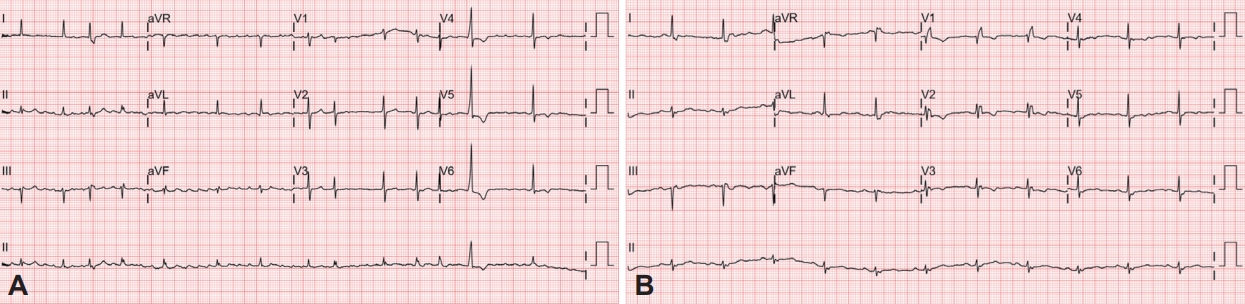
A 77-year-old woman with a history of hypertension, angina, AF, and chronic kidney disease was evaluated for snoring, witnessed apnea, and nonrestorative sleep. The Epworth Sleepiness Scale score and Pittsburgh Sleep Quality Index were 12 and 7, respectively, indicating daytime sleepiness and poor sleep quality. On physical examination, the patient was obese with a body mass index of 27.6 kg/m2 (weight 68.0 kg, height 157.3 cm). Her neck was short and thick (neck circumference, 38.0 cm). Electrocardiography confirmed the presence of AF with a ventricular rate of 97 beats per minute (range: 66–153 beats per minute) (Fig. 1A). Laboratory test results, including electrolyte levels and thyroid function tests, indicated no apparent cause for AF. Echocardiography showed a left ventricular ejection fraction of 68.8%, normal right ventricular systolic function, left atrial cavity volume of 118 mL, and left atrial volume index of 69.8 mL/m2, consistent with atrial dilatation. Split-night polysomnography (PSG) was performed to evaluate the suspected OSA. The split-night protocol was performed only when the following criteria were met: 1) apnea-hypopnea index (AHI) ≥20 is observed during a minimum of 2 hours on the diagnostic part; 2) at least 3 hours remain for CPAP titration [4]. The PSG was scored manually according to the American Academy of Sleep Medicine standard criteria for sleep scoring. Apnea was defined as a ≥90% drop in airflow from baseline for ≥10 s with ongoing respiratory efforts. Hypopnea was scored when the airflow signal reduced ≥30% from baseline for ≥10 s with ≥3% oxygen desaturation. AHI is defined as the average number of apneas and hypopneas per hour of sleep. The diagnostic part of the PSG revealed severe OSA, with a high AHI of 34.0/h (hypopnea index of 31.2/h and obstructive apnea index of 2.8/h). The duration of the longest obstructive apnea was 34.9 s and the lowest oxygen saturation (SaO2) was 82%. In the CPAP titration, pressure was manually titrated from 4 cm H2O to an optimal setting of 9 cm H2O (Fig. 2A). The diagnostic part of PSG showed sustained AF with rapid ventricular responses. Although AF remained persistent under a CPAP pressure of 4–8 cm H2O, the AF converted to normal sinus rhythm at a pressure of 9 cm H2O. This was maintained during the night and the patient’s heart rate was also normal (Fig. 2B and C). Moreover, the fluctuating hypoxemia and irregular heart rhythm improved at a pressure of 9 cm H2O. The patient showed good adherence with CPAP use (average hours per night of PAP use: 6.6 h; percentage of nights with ≥4 h PAP use: 100%; average residual AHI: 3.8/h) and reported reduced daytime sleepiness and better sleep quality. One month after CPAP initiation, the patient had a normal sinus rhythm with a regular rhythm at a rate of 63 beats per minute on CPAP (Fig. 1B). Ethics approval for the study was obtained from the Institutional Review Board of Seoul National University Bundang Hospital (IRB No. B-2207-770-701). Informed consent was exempt from IRB.
DISCUSSION
AF is the most common cardiac arrhythmia and the leading cause of cardiovascular morbidity and mortality. The prevalence of AF varies from 0.9%–2.7% based on the geographical region, and a recent cohort study reported the prevalence of AF in Korean population to be 0.67% [5]. Current known risk factors for AF include older age, male sex, obesity, hypertension, diabetes mellitus, and heart disease. Recently, OSA has been found to be highly prevalent among patients with AF. This indicates that OSA may play a role in the development and maintenance of AF. The Sleep Heart Health Study reported that patients with severe OSA had AF 4-times more often than those without sleep apnea (4.8% vs. 0.9%) [6]. The candidate sleep apnea-related arrhythmogenic mechanisms include hypoxic burden, intrathoracic pressure shifts, inflammation, and autonomic nervous system instability, leading to structural and electrical remodeling of the heart [7]. Repetitive hypoxemia causes oxidative stress, systemic inflammation, and sympathetic surges, inducing vasoconstriction, hypertension, and tachycardia. This increased stress on the myocardium ultimately leads to adverse myocardial remodeling. Uninhibited parasympathetic activity gives rise to significant paroxysmal bradycardia which leads to a short atrial effective refractory period and renders the atrium more vulnerable to AF [8]. The sudden swing in the intrathoracic pressure causes the atrial chamber to stretch thereby predisposing the heart to electrophysiological and structural alterations [9].
Although it remains controversial whether the use of CPAP in OSA patients influences the amount, duration, or recurrence of AF, there is increasing evidence of improved arrhythmia control with CPAP therapy. OSA patients treated with CPAP had a lower rate or recurrence of AF at 1 year than those not treated with CPAP (42% vs. 82%) [10]. The mechanism by which CPAP treatment decreases the risk of AF recurrence is not completely understood. However, the reduction of hypoxic burden with CPAP might reverse structural and electrical changes in the heart. Additionally, CPAP improves hypertension, diabetes, and obesity, which are overlapping risk factors for OSA and AF. CPAP may mitigate the risk of AF episodes by preventing and reversing the consequences of OSA and improving the various risk factors associated with OSA and AF.
This case report described the conversion of AF to normal sinus rhythm with optimized CPAP treatment in an older female patient with severe OSA. As mentioned, a potential mechanism underlying the conversion of AF to normal sinus rhythm may be the acute effect of CPAP by the abolition of negative intrathoracic pressure swings and reduction of nocturnal blood pressure and heart rate, resulting in the alleviation of myocardial wall stress. Although the degree of hypoxemia was not profound in our patient (SaO2 range, 90%–94%), the reversal of hypoxemia with CPAP may also be another possible mechanism. The main limitation of this report was that the long-term (beyond one month) effect of CPAP on AF recurrence was not investigated.
In conclusion, our case provides evidence that, in patients with severe OSA and AF, the optimal level of CPAP could lead to the immediate conversion of AF to normal sinus rhythm, without cardioversion and catheter ablation. We suggest that CPAP treatment may be a potential therapy for patients with AF in addition to antiarrhythmic drugs, cardioversion, and ablation. Furthermore, clinicians should consider the coexistence of OSA and AF, and both should be treated in parallel.